Facial Cleansing Wipes
43116-041
36f7677a-ec73-e677-e063-6294a90ad480
HUMAN OTC DRUG LABEL
Jun 7, 2025
Shenzhen Shierjie Biological Engineering Co., LTD
DUNS: 547610261
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Facial Cleansing Wipes
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (17)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL

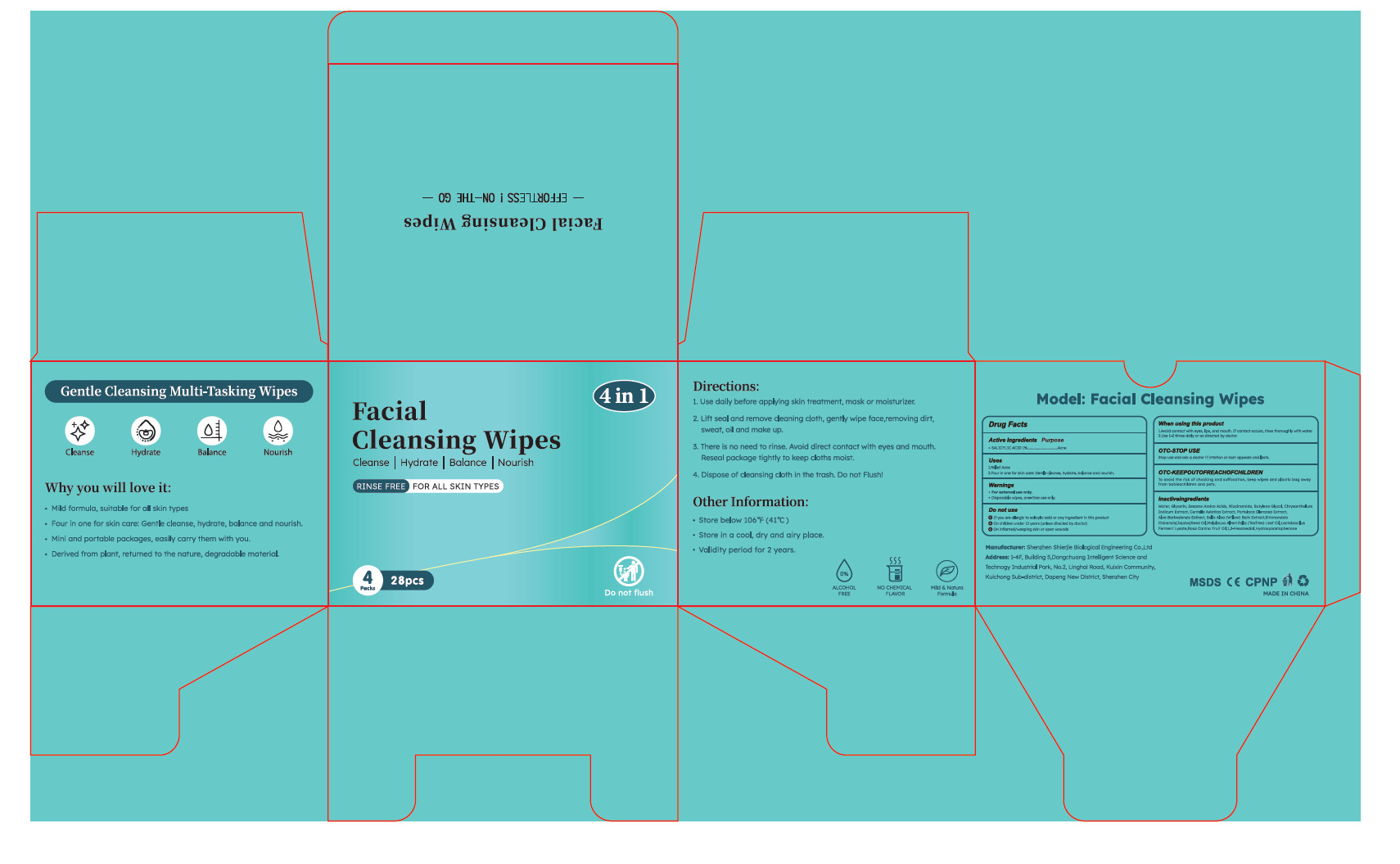
INDICATIONS & USAGE SECTION
Use
1.Relief Acne
2.Four in one for skin care: Gentle cleanse, hydrate, balance and nourish.
WARNINGS SECTION
Warnings
●For external use only.
●Disposable wipes, one-time use only.
OTC - ACTIVE INGREDIENT SECTION
Active Ingredient
SAL ICYLIC ACID 1%
OTC - PURPOSE SECTION
Purpose
Acne
OTC - DO NOT USE SECTION
Do not use
1. If you are allergic to salicylic acid or any ingredient in this product
2. On children under 12 years (unless directed by doctor)
3. On inflamed/weeping skin or open wounds
OTC - WHEN USING SECTION
When Using
1.Avoid contact with eyes, lips, and mouth. If contact occurs, rinse
thoroughly with water.
2.Use 1-2 times daily or as directed by doctor.
OTC - STOP USE SECTION
Stop Use
Stop use and ask a doctor if irtation or rash appears and lasts.
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
Keep Oot Of Reach Of Children
To avid the isk of chocking and ufocationnn keep wipes and plastic bag awoy
from babieschildren and pets.
DOSAGE & ADMINISTRATION SECTION
Directions
1. Use daily before applying skin treatment, mask or moisturizer.
2. Lift seal and remove cleaning cloth, gently wipe face,removing dirt,
sweat, oil and make up.
3. There is no need to rinse. Avoid direct contact with eyes and mouth.
Reseal package tightly to keep cloths moist.
4. Dispose of cleansing cloth in the trash. Do not Flush!
STORAGE AND HANDLING SECTION
Other information
●Store below 106°F (41°C )
●Store in a cool, dry and airy place.
●Validity period for 2 years.
INACTIVE INGREDIENT SECTION
Inactive ingredients
Water, Glycerin, Sesame Amino Acids, Niacinamide, Butylene Glycol,
Chrysanthellum
Indicum Extract, Centella Asiatica Extract, Portulaca Oleracea Extract,
Aloe Barbadensis Extract, Salix Alba (Willow) Bark Extract Simmondsia
Chinensis(Jojoba)Seed Oil,Melaleuca Alternifolia (TeaTree) Leaf
Oil,Lactobacillus
Ferment Lysate,Rosa Canina Fruit Oil,1,2-Hexanediol,Hydroxyacetophenone
