Atropine Sulfate
These highlights do not include all the information needed to use ATROPINE SULFATE INJECTION safely and effectively. See full prescribing information for ATROPINE SULFATE INJECTION. ATROPINE SULFATE INJECTION, for intravenous use Initial U.S. Approval: 1960
d4839e54-b350-4643-8e6e-f0437f5349d6
HUMAN PRESCRIPTION DRUG LABEL
Apr 17, 2025
Henry Schein, Inc.
DUNS: 012430880
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Atropine Sulfate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (1)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
SAMPLE PACKAGE LABEL

INDICATIONS & USAGE SECTION
1 INDICATIONS AND USAGE
Atropine Sulfate Injection is indicated for temporary blockade of severe or
life-threatening muscarinic
effects, e.g., as an antisialagogue, an antivagal agent, an antidote for
organophosphorus or muscarinic
mushroom poisoning, and to treat bradyasystolic cardiac arrest.
Atropine is a muscarinic antagonist indicated for temporary blockade of severe or life-threatening muscarinic effects. (1) (1)
CONTRAINDICATIONS SECTION
4 CONTRAINDICATIONS
None.
None. (4)
WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Tachycardia
When the recurrent use of atropine is essential in patients with coronary
artery disease, the total dose
should be restricted to 2 to 3 mg (maximum 0.03 to 0.04 mg/kg) to avoid the
detrimental effects of
atropine-induced tachycardia on myocardial oxygen demand.
5.2 Acute Glaucoma
Atropine may precipitate acute glaucoma.
5.3 Pyloric Obstruction
Atropine may convert partial organic pyloric stenosis into complete
obstruction.
5.4 Complete Urinary Retention
Atropine may lead to complete urinary retention in patients with prostatic
hypertrophy.
5.5 Viscid Plugs
Atropine may cause inspissation of bronchial secretions and formation of
viscid plugs in patients with
chronic lung disease.
• Tachycardia (5.1)
• Glaucoma (5.2)
• Pyloric obstruction (5.3)
• Worsening urinary retention (5.4)
• Viscid bronchial plugs (5.5) (5)
ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
The following adverse reactions have been identified during post-approval use
of atropine sulfate.
Because these reactions are reported voluntarily from a population of
uncertain size, it is not always
possible to reliably estimate their frequency or establish a causal
relationship to drug exposure.
Most of the side effects of atropine are directly related to its
antimuscarinic action. Dryness of the mouth,
blurred vision, photophobia and tachycardia commonly occur. Anhidrosis can
produce heat intolerance.
Constipation and difficulty in micturition may occur in elderly patients.
Occasional hypersensitivity
reactions have been observed, especially skin rashes which in some instances
progressed to exfoliation.
Most adverse reactions are directly related to atropine’s antimuscarinic
action.
Dryness of the mouth, blurred vision, photophobia and tachycardia commonly
occur with chronic administration of therapeutic doses. (6) (6)
To report SUSPECTED ADVERSE REACTIONS, contact Hospira, Inc. (6)
at 1-800-441-4100, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. (6)
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
7.1 Mexiletine
Atropine Sulfate Injection decreased the rate of mexiletine absorption without
altering the relative oral
bioavailability; this delay in mexiletine absorption was reversed by the
combination of atropine and
intravenous metoclopramide during pretreatment for anesthesia.
Mexiletine Decreases rate of mexiletine absorption. (7.1)
Revised: 11/2020 (7)
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
2.1 General Administration
Parenteral drug products should be inspected visually for particulate matter
and discoloration prior to
administration, whenever solution and container permit. Do not administer
unless solution is clear and
seal is intact. Each syringe is intended for single dose only. Discard unused
portion.
For intravenous administration.
Titrate based on heart rate, PR interval, blood pressure and symptoms.
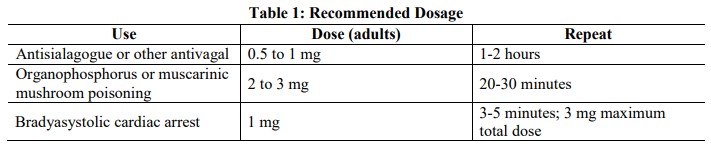
2.2 Adult Dosage
2.3 Pediatric Dosage
Dosing in pediatric populations has not been well studied. Usual initial dose
is 0.01 to 0.03 mg/kg.
2.4 Dosing in Patients with Coronary Artery Disease
Limit the total dose of atropine sulfate to 0.03 mg/kg to 0.04 mg/kg [see Warnings and Precautions (5.1)]
• For intravenous administration (2.1)
• Titrate according to heart rate, PR interval, blood pressure and
symptoms (2.1)
• Adult dosage
- Antisialagogue or for antivagal effects: Initial single dose of 0.5 mg to
1 mg (2.2)
- Antidote for organophosphorus or muscarinic mushroom poisoning:
Initial single dose of 2 mg to 3 mg, repeated every 20-30 minutes (2.2)
- Bradyasystolic cardiac arrest: 1 mg dose, repeated every 3-5 minutes if
asystole persists (2.2)
• Patients with Coronary Artery Disease: Limit the total dose to 0.03 mg/kg
to 0.04 mg/kg (2.4) (2)
DOSAGE FORMS & STRENGTHS SECTION
3 DOSAGE FORMS AND STRENGTHS
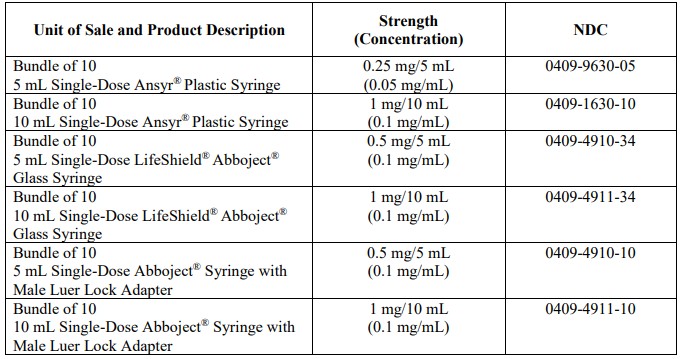
Injection: 0.05 mg/mL and 0.1 mg/mL in Ansyr® Plastic Syringe.
Injection: 0.1 mg/mL in LifeShield® Abboject® Glass Syringe.
Injection: 0.1 mg/mL in Abboject® Syringe with Male Luer Lock Adapter.
• 0.05 mg/mL injection in Ansyr® Plastic Syringe (3)
• 0.1 mg/mL injection in Ansyr® Plastic Syringe (3)
• 0.1 mg/mL injection in LifeShield® Abboject® Glass Syringe (3)
• 0.1 mg/mL injection in Abboject® Syringe with Male Luer Lock Adapter (3) (3)
USE IN SPECIFIC POPULATIONS SECTION
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Animal reproduction studies have not been conducted with atropine. It also is
not known whether atropine
can cause fetal harm when given to a pregnant woman or can affect reproduction
capacity.
8.3 Nursing Mothers
Trace amounts of atropine was found in breast milk. The clinical impact of
this is not known.
8.4 Pediatric Use
Recommendations for use in pediatric patients are not based on clinical
trials.
8.5 Geriatric Use
An evaluation of current literature revealed no clinical experience
identifying differences in response
between elderly and younger patients. In general, dose selection for an
elderly patient should be cautious,
usually starting at the low end of the dosing range, reflecting the greater
frequency of decreased hepatic,
renal, or cardiac function, and of concomitant disease or other drug therapy.
OVERDOSAGE SECTION
10 OVERDOSAGE
Excessive dosing may cause palpitation, dilated pupils, difficulty in
swallowing, hot dry skin, thirst,
dizziness, restlessness, tremor, fatigue and ataxia. Toxic doses lead to
restlessness and excitement,
hallucinations, delirium and coma. Depression and circulatory collapse occur
only with severe
intoxication. In such cases, blood pressure declines and death due to
respiratory failure may ensue
following paralysis and coma.
The fatal adult dose of atropine is not known. In pediatric populations, 10 mg
or less may be fatal.
In the event of toxic overdosage, a short acting barbiturate or diazepam may
be given as needed to control
marked excitement and convulsions. Large doses for sedation should be avoided
because central
depressant action may coincide with the depression occurring late in atropine
poisoning. Central
stimulants are not recommended.
Physostigmine, given as an atropine antidote by slow intravenous injection of
1 to 4 mg (0.5 to 1 mg in
pediatric populations), rapidly abolishes delirium and coma caused by large
doses of atropine. Since
physostigmine is rapidly destroyed, the patient may again lapse into coma
after one to two hours, and
repeated doses may be required.
Artificial respiration with oxygen may be necessary. Ice bags and alcohol
sponges help to reduce fever,
especially in pediatric populations.
Atropine is not removed by dialysis
DESCRIPTION SECTION
11 DESCRIPTION
Atropine Sulfate Injection, USP is a sterile, nonpyrogenic isotonic solution
of atropine sulfate
monohydrate in water for injection with sodium chloride sufficient to render
the solution isotonic. It is
administered parenterally by intravenous injection.
Each milliliter (mL) contains 0.1 mg (adult strength) or 0.05 mg (pediatric
strength) of atropine sulfate
monohydrate equivalent to 0.083 mg (adult strength) or 0.042 mg (pediatric
strength) of atropine, and
sodium chloride, 9 mg. May contain sodium hydroxide and/or sulfuric acid for
pH adjustment
0.308 mOsmol/mL (calc.). pH 3.0 to 6.5.
Sodium chloride added to render the solution isotonic for injection of the
active ingredient is present in
amounts insufficient to affect serum electrolyte balance of sodium (Na+
) and chloride (Cl-
) ions.
The solution contains no bacteriostat, antimicrobial agent or added buffer
(except for pH adjustment) and
is intended for use only as a single-dose injection. When smaller doses are
required the unused portion
should be discarded.
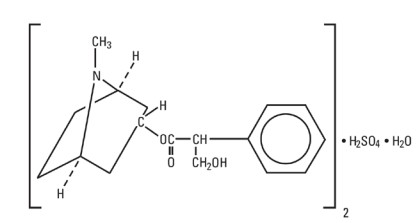
Atropine Sulfate, USP is chemically designated 1α H, 5α H-Tropan-3-α-ol
(±)-tropate (ester), sulfate (2:1)
(salt) monohydrate, (C17H23NO3)2 H2SO4 H2O, colorless crystals or white
crystalline powder very
soluble in water. It has the following structural formula:
Atropine, a naturally occurring belladonna alkaloid, is a racemic mixture of
equal parts of d- and
1-hyocyamine, whose activity is due almost entirely to the levo isomer of the
drug.
Sodium Chloride, USP is chemically designated NaCl, a white crystalline powder
freely soluble in water.
The Ansyr® syringe is molded from a specially formulated polypropylene. Water
permeates from inside
the container at an extremely slow rate which will have an insignificant
effect on solution concentration
over the expected shelf life. Solutions in contact with the plastic container
may leach out certain chemical
components from the plastic in very small amounts; however, biological testing
was supportive of the
safety of the syringe material
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Atropine is an antimuscarinic agent since it antagonizes the muscarine-like
actions of acetylcholine and
other choline esters.
Atropine inhibits the muscarinic actions of acetylcholine on structures
innervated by postganglionic
cholinergic nerves, and on smooth muscles which respond to endogenous
acetylcholine but are not so
innervated. As with other antimuscarinic agents, the major action of atropine
is a competitive or
surmountable antagonism which can be overcome by increasing the concentration
of acetylcholine at
receptor sites of the effector organ (e.g., by using anticholinesterase agents
which inhibit the enzymatic
destruction of acetylcholine). The receptors antagonized by atropine are the
peripheral structures that are
stimulated or inhibited by muscarine (i.e., exocrine glands and smooth and
cardiac muscle). Responses to
postganglionic cholinergic nerve stimulation also may be inhibited by atropine
but this occurs less readily
than with responses to injected (exogenous) choline esters.
12.2 Pharmacodynamics
Atropine-induced parasympathetic inhibition may be preceded by a transient
phase of stimulation,
especially on the heart where small doses first slow the rate before
characteristic tachycardia develops due
to paralysis of vagal control. Atropine exerts a more potent and prolonged
effect on heart, intestine and
bronchial muscle than scopolamine, but its action on the iris, ciliary body
and certain secretory glands is
weaker than that of scopolamine. Unlike the latter, atropine in clinical doses
does not depress the central
nervous system but may stimulate the medulla and higher cerebral centers.
Although mild vagal excitation
occurs, the increased respiratory rate and (sometimes) increased depth of
respiration produced by atropine
are more probably the result of bronchiolar dilatation. Accordingly, atropine
is an unreliable respiratory
stimulant and large or repeated doses may depress respiration.
Adequate doses of atropine abolish various types of reflex vagal cardiac
slowing or asystole. The drug
also prevents or abolishes bradycardia or asystole produced by injection of
choline esters,
anticholinesterase agents or other parasympathomimetic drugs, and cardiac
arrest produced by stimulation
of the vagus. Atropine also may lessen the degree of partial heart block when
vagal activity is an etiologic
factor. In some patients with complete heart block, the idioventricular rate
may be accelerated by atropine;
in others, the rate is stabilized. Occasionally a large dose may cause
atrioventricular (A-V) block and
nodal rhythm.
Atropine Sulfate Injection in clinical doses counteracts the peripheral
dilatation and abrupt decrease in
blood pressure produced by choline esters. However, when given by itself,
atropine does not exert a
striking or uniform effect on blood vessels or blood pressure. Systemic doses
slightly raise systolic and
lower diastolic pressures and can produce significant postural hypotension.
Such doses also slightly
increase cardiac output and decrease central venous pressure. Occasionally,
therapeutic doses dilate
cutaneous blood vessels, particularly in the “blush” area (atropine flush) and
may cause atropine “fever”
due to suppression of sweat gland activity in infants and small children.
The effects of intravenous atropine on heart rate (maximum heart rate) and
saliva flow (minimum flow)
after intravenous administration (rapid, constant infusion over 3 min.) are
delayed by 7 to 8 minutes after
drug administration and both effects are non-linearly related to the amount of
drug in the peripheral
compartment. Changes in plasma atropine levels following intramuscular
administration (0.5 to
4 mg doses) and heart rate are closely overlapped but the time course of the
changes in atropine levels and
behavioral impairment indicates that pharmacokinetics is not the primary rate-
limiting mechanism for the
central nervous system effect of atropine.
12.3 Pharmacokinetics
Atropine disappears rapidly from the blood following injection and is
distributed throughout the body.
Exercise, both prior to and immediately following intramuscular administration
of atropine, significantly
increases the absorption of atropine due to increased perfusion in the muscle
and significantly decreases
the clearance of atropine. The pharmacokinetics of atropine is nonlinear after
intravenous administration
of 0.5 to 4 mg. Atropine’s plasma protein binding is about 44% and saturable
in the 2-20 μg/mL
concentration range. Atropine readily crosses the placental barrier and enters
the fetal circulation, but is
not found in amniotic fluid. Much of the drug is destroyed by enzymatic
hydrolysis, particularly in the
liver; from 13 to 50% is excreted unchanged in the urine. Traces are found in
various secretions, including
milk. The major metabolites of atropine are noratropine, atropin-n-oxide,
tropine, and tropic acid. The
metabolism of atropine is inhibited by organophosphate pesticides.
Specific Populations
The elimination half-life of atropine is more than doubled in children under
two years and the elderly
(>65 years old) compared to other age groups. There is no gender effect on the
pharmacokinetics and
pharmacodynamics (heart rate changes) of atropine.
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Studies have not been performed to evaluate the carcinogenic or mutagenic
potential of atropine or its
potential to affect fertility adversely.
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
Atropine Sulfate Injection, USP is supplied in single-dose syringes as follows:
Store at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C and
30°C (59°F and 86°F).
[See USP Controlled Room Temperature.]
|
From Original Manufacturer/Distributor's NDC and Unit of Sale |
To Henry Schein Repackaged Product NDC and Unit of Sale |
Total Strength/Total Volume (Concentration) per unit |
|
NDC 0409-9630-05 |
NDC 0404-9823-05 |
Concentration: 0.05 mg/mL |
Distributed by Hospira, Inc., Lake Forest, IL 60045 USA
Abboject® is a trademark of Abbott Laboratories.
LifeShield® is the trademark of ICU Medical, Inc. and is used under license.
LAB-1041-4.2
