Sildenafil
These highlights do not include all the information needed to use SILDENAFIL TABLETS safely and effectively. See full prescribing information for SILDENAFIL TABLETS. SILDENAFIL tablets, for oral use Initial U.S. Approval: 1998
333ca682-931f-4edb-95d6-2edbd87631cf
HUMAN PRESCRIPTION DRUG LABEL
Nov 30, 2023
Quallent Pharmaceuticals Health LLC
DUNS: 815564528
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Sildenafil
PRODUCT DETAILS
INGREDIENTS (12)
Drug Labeling Information
INDICATIONS & USAGE SECTION
1 INDICATIONS AND USAGE
Adults
Sildenafil tablets, USP are indicated for the treatment of pulmonary arterial hypertension (PAH) (World Health Organization [WHO] Group I) in adults to improve exercise ability and delay clinical worsening [see Clinical Studies (14)].
Pediatric use information is approved for Viatris Specialty LLC's, REVATIO (sildenafil) tablets. However, due to Viatris Specialty LLC's marketing exclusivity rights, this drug product is not labeled with that information.
CONTRAINDICATIONS SECTION
4 CONTRAINDICATIONS
Sildenafil tablets are contraindicated in patients with:
- Concomitant use of organic nitrates in any form, either regularly or intermittently, because of the greater risk of hypotension [see Warnings and Precautions (5.1)].
- Concomitant use of riociguat, a guanylate cyclase stimulator. Phosphodiesterase-5 (PDE-5) inhibitors, including sildenafil, may potentiate the hypotensive effects of riociguat.
- Known hypersensitivity to sildenafil or any component of the tablet. Hypersensitivity, including anaphylactic reaction, anaphylactic shock and anaphylactoid reaction, has been reported in association with the use of sildenafil.
- Use with organic nitrates or riociguat. (4)
- History of hypersensitivity reaction to sildenafil or any component of the tablet. (4)
ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
The following serious adverse events are discussed elsewhere in the labeling:
- Hypotension [see Warnings and Precautions (5.1)]
- Vision loss [see Warnings and Precautions (5.4)]
- Hearing loss [see Warnings and Precautions (5.5)]
- Priapism [see Warnings and Precautions (5.7)]
- Vaso-occlusive Crisis in Patients with Pulmonary Hypertension Secondary to Sickle Cell Disease [see Warnings and Precautions (5.8)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In a 12-week, placebo-controlled clinical study and an open-label extension study (SUPER-1) in 277 sildenafil tablets-treated adults with PAH (WHO Group I) [see Clinical Studies (14)] the adverse reactions that were reported by at least 10% of sildenafil tablets-treated patients in any dosing group, and were more frequent in sildenafil tablets-treated patients than in placebo-treated patients are shown in Table 1. Adverse reactions were generally transient and mild to moderate in nature. The overall frequency of discontinuation in sildenafil tablets-treated patients was 3% (20 mg and 40 mg three times a day) and 8% (80 mg three times a day). The overall frequency of discontinuation for placebo was 3%.****
Table 1. Most Common Adverse Reactions in Patients Treated with Sildenafil tablets 20 mg, 40 mg, 80 mg and Placebo three times per day in SUPER-1 (More Frequent in Sildenafil tablets- Treated Patients than Placebo- Treated Patients)|
Sildenafil tablets |
Sildenafil |
Sildenafil |
Placebo (n = 70) | |
|
Headache |
46% |
42% |
49% |
39% |
|
Flushing |
10% |
9% |
16% |
4% |
|
Pain in Limb |
7% |
15% |
9% |
6% |
|
Myalgia |
7% |
6% |
14% |
4% |
|
Back Pain |
13% |
13% |
9% |
11% |
|
Dyspepsia |
13% |
8% |
13% |
7% |
|
Diarrhea |
9% |
12% |
10% |
6% |
In a placebo-controlled fixed dose titration study (PACES-1) of sildenafil tablets (starting with recommended dose of 20 mg and increased to 40 mg and then 80 mg all three times a day) as an adjunct to intravenous epoprostenol in patients with PAH, no new safety issues were identified except for edema, which occurred in 25% of subjects in the combined sildenafil tablets + epoprostenol group compared with 13% of subjects in the epoprostenol group [see Clinical Studies (14)].
Pediatric use information is approved for Viatris Specialty LLC's, REVATIO (sildenafil) tablets. However, due to Viatris Specialty LLC's marketing exclusivity rights, this drug product is not labeled with that information.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use of sildenafil (marketed for both PAH and erectile dysfunction). Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Cardiovascular Events
In postmarketing experience with sildenafil at doses indicated for erectile dysfunction, serious cardiovascular, cerebrovascular, and vascular events, including myocardial infarction, sudden cardiac death, ventricular arrhythmia, cerebrovascular hemorrhage, transient ischemic attack, hypertension, pulmonary hemorrhage, and subarachnoid and intracerebral hemorrhages have been reported in temporal association with the use of the drug. Most, but not all, of these patients had preexisting cardiovascular risk factors. Many of these events were reported to occur during or shortly after sexual activity, and a few were reported to occur shortly after the use of sildenafil without sexual activity. Others were reported to have occurred hours to days after use concurrent with sexual activity. It is not possible to determine whether these events are related directly to sildenafil, to sexual activity, to the patient’s underlying cardiovascular disease, or to a combination of these or other factors.
Nervous System
Seizure, seizure recurrence
Ophthalmologic
NAION [see Warnings and Precautions (5.4), and Patient Counseling Information (17)].
Adults: Headache, dyspepsia, flushing, pain in limb, myalgia, back pain and diarrhea. (6.1, 6.2)
To report SUSPECTED ADVERSE REACTIONS, contact Quallent Pharmaceuticals Health LLC at 1-877-605-7243 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
Nitrates
Concomitant use of sildenafil tablets with nitrates in any form is contraindicated [see Contraindications (4)].
Strong CYP3A Inhibitors
Concomitant use of sildenafil tablets with strong CYP3A inhibitors is not recommended [see Clinical Pharmacology (12.3)].
Moderate-to-Strong CYP3A Inducers
Concomitant use of sildenafil tablets with moderate-to-strong CYP3A inducers (such as bosentan) decreases the sildenafil exposure. Dose up-titration of sildenafil tablets may be needed when initiating treatment with moderate-to- strong CYP3A inducers. Reduce the dose of sildenafil tablets to 20 mg three times a day when discontinuing treatment with moderate-to-strong CYP3A inducers [see Clinical Pharmacology (12.3) and Clinical Studies (14)].
- Use with strong CYP3A inhibitors: Not recommended. (7, 12.3)
- Concomitant PDE-5 inhibitors: Avoid use with Viagra® or other PDE-5 inhibitors. (5.6)
Pediatric use information is approved for Viatris Specialty LLC's, REVATIO (sildenafil) tablets. However, due to Viatris Specialty LLC's marketing exclusivity rights, this drug product is not labeled with that information.
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage in Adults
Oral Dosage
The recommended dosage of sildenafil tablets are 20 mg three times a day. [see Clinical Studies (14)].
Pediatric use information is approved for Viatris Specialty LLC's, REVATIO (sildenafil) tablets. However, due to Viatris Specialty LLC's marketing exclusivity rights, this drug product is not labeled with that information.
DOSAGE FORMS & STRENGTHS SECTION
3 DOSAGE FORMS AND STRENGTHS
Sildenafil Tablets
White, film-coated, circular, biconvex tablets debossed with 'SC' on one side and '20' on other side containing sildenafil citrate equivalent to 20 mg of sildenafil.
DESCRIPTION SECTION
11 DESCRIPTION
Sildenafil, phosphodiesterase-5 (PDE-5) inhibitor, is the citrate salt of sildenafil, a selective inhibitor of cyclic guanosine monophosphate (cGMP)-specific phosphodiesterase type-5 (PDE-5). Sildenafil is also marketed as VIAGRA® for erectile dysfunction.
Sildenafil citrate is designated chemically as 1-[[3-(6,7-dihydro-1-methyl-7-oxo-3-propyl-1H-pyrazolo [4,3-d] pyrimidin-5-yl)-4-ethoxyphenyl] sulfonyl]-4-methylpiperazine citrate and has the following structural formula:
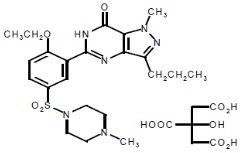
Sildenafil citrate, USP is a white to off-white crystalline powder with a solubility of 3.5 mg/mL in water and a molecular weight of 666.7.
Sildenafil Tablets: Sildenafil tablets, USP is formulated as white, film- coated circular, biconvex tablets for oral administration. Each tablet contains sildenafil citrate USP equivalent to 20 mg of sildenafil. In addition to the active ingredient, sildenafil citrate USP, each tablet contains the following inactive ingredients: anhydrous dibasic calcium phosphate, colloidal silicon dioxide, croscarmellose sodium, magnesium stearate, microcrystalline cellulose, polyethylene glycol, titanium dioxide, polyvinyl alcohol and talc.
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Sildenafil was not carcinogenic when administered to rats for up to 24 months at 60 mg/kg/day, a dose resulting in total systemic exposure (AUC) to unbound sildenafil and its major metabolite 33- and 37-times, for male and female rats, respectively, the human exposure at the RHD of 20 mg three times a day. Sildenafil was not carcinogenic when administered to male and female mice for up to 21 and 18 months, respectively, at doses up to a maximally tolerated level of 10 mg/kg/day, a dose equivalent to the RHD on a mg/m2 basis.
Sildenafil was negative in in vitro bacterial and Chinese hamster ovary cell assays to detect mutagenicity, and in vitro human lymphocytes and in vivo mouse micronucleus assays to detect clastogenicity.
There was no impairment of fertility in male or female rats given up to 60 mg sildenafil/kg/day, a dose producing a total systemic exposure (AUC) to unbound sildenafil and its major metabolite of 19- and 38- times for males and females, respectively, the human exposure at the RHD of 20 mg three times a day.
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
- Inform patients of contraindication of sildenafil tablets with regular and/or intermittent use of organic nitrates.
- Inform patients that sildenafil is also marketed as VIAGRA for erectile dysfunction. Advise patients taking sildenafil tablets not to take VIAGRA or other PDE-5 inhibitors.
- Advise patients to seek immediate medical attention for a sudden loss of vision in one or both eyes while taking sildenafil tablets. Such an event may be a sign of NAION.
- Advise patients to seek prompt medical attention in the event of sudden decrease or loss of hearing while taking sildenafil tablets. These events may be accompanied by tinnitus and dizziness.
Trademarks are the property of their respective owners.
Manufactured by:
Ajanta Pharma Limited
India
Manufactured for:
Quallent Pharmaceuticals Health LLC
Grand Cayman, Cayman Islands

