UROCIT-K
Urocit ®-K (Potassium Citrate) Extended Release Tablets for Oral Use These highlights do not include all the information needed to use Urocit -K safely and effectively. See full prescribing information for Urocit -K. Initial U.S. Approval: 1985
72cdea1b-2240-41db-987d-86d5c6aaa978
HUMAN PRESCRIPTION DRUG LABEL
Aug 13, 2025
Mission Pharmacal Company
DUNS: 008117095
Products 3
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
potassium citrate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (3)
potassium citrate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (3)
potassium citrate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (3)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 15 mEq Bottle Label
NDC0178-0615-01
UROCIT**®-K15 mEq****(Potassium Citrate)**Extended-release tablet
15 mEq (1620 mg) per tablet
Rx only
100 Tablets
Mission Pharmacal Company
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
7.1 Potential Effects of Potassium Citrate on Other Drugs
Potassium-sparing Diuretics:Concomitant administration of Urocit-K and a potassium-sparing diuretic (such as triamterene, spironolactone or amiloride) should be avoided since the simultaneous administration of these agents can produce severe hyperkalemia.
7.2 Potential Effects of Other Drugs on Potassium Citrate
Drugs that slow gastrointestinal transit time:These agents (such as anticholinergics) can be expected to increase the gastrointestinal irritation produced by potassium salts.
7.3 Renin-Angiotensin-Aldosterone System Inhibitors
Drugs that inhibit the renin-angiotensin-aldosterone system (RAAS) including angiotensin converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), spironolactone, eplerenone, or aliskiren produce potassium retention by inhibiting aldosterone production. Closely monitor potassium in patients receiving concomitant RAAS therapy.
7.4 Nonsteroidal Anti-Inflammatory Drugs (NSAIDs)
NSAIDs may produce potassium retention by reducing renal synthesis of prostagladin E and impairing the renin-angiotensin system. Closely monitor potassium in patients on concomitant NSAIDs.
The following drug interactions may occur with potassium citrate: (7)
- Potassium-sparing diuretics: concomitant administration should be avoided since the simultaneous administration of these agents can produce severe hyperkalemia (7.1)
- Drugs that slow gastrointestinal transit time: These agents (such as anticholinergics) can be expected to increase the gastrointestinal irritation produced by potassium salts (7.2)
- Renin-angiotensin-aldosterone inhibitors: Monitor for hyperkalemia (7.3)
- Nonsteroidal Anti-inflammatory drugs (NSAIDs) monitor for hyperkalemia (7.4)
USE IN SPECIFIC POPULATIONS SECTION
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Animal reproduction studies have not been conducted. It is also not known whether Urocit-K can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Urocit-K should be given to a pregnant woman only if clearly needed.
8.3 Nursing Mothers
The normal potassium ion content of human milk is about 13 mEq/L. It is not known if Urocit-K has an effect on this content. Urocit-K should be given to a woman who is breastfeeding only if clearly needed.
8.4 Pediatric Use
Safety and effectiveness in children have not been established.
(8)
- Pregnant women: Animal reproduction studies have not been conducted. It is not known whether Urocit-K can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Urocit-K should be given to a pregnant woman only if clearly needed (8.1)
- Nursing mothers: The normal potassium ion content of human milk is about 13 mEq/L. It is not known if Urocit-K has an effect on this content. Urocit-K should be given to a woman who is breastfeeding only if clearly needed (8.3)
- Pediatric Use: Safety and effectiveness in children have not been established (8.4)
See 17 for PATIENT COUNSELING INFORMATION (8)
** Revised 12/2021** (8)
(8)
(8)
DESCRIPTION SECTION
11 DESCRIPTION
Urocit-K is a citrate salt of potassium. Its empirical formula is K3C6H507 • H20, and it has the following chemical structure:
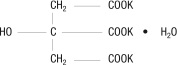
Urocit-K yellowish to tan, oral wax-matrix tablets, contain 5 mEq (540 mg) potassium citrate, 10 mEq (1080 mg) potassium citrate and 15 mEq (1620 mg) potassium citrate each. Inactive ingredients include carnauba wax and magnesium stearate.
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Tell patients to take each dose without crushing, chewing or sucking the tablet.
Tell patients to take this medicine only as directed. This is especially important if the patient is also taking both diuretics and digitalis preparations.
Tell patients to check with the doctor if there is trouble swallowing tablets or if the tablet seems to stick in the throat.
Tell patients to check with the doctor at once if tarry stools or other evidence of gastrointestinal bleeding is noticed. Tell patients that their doctor will perform regular blood tests and electrocardiograms to ensure safety.
L061001R0521
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
The pivotal Urocit-K trials were non-randomized and non-placebo controlled where dietary management may have changed coincidentally with pharmacological treatment. Therefore, the results as presented in the following sections may overstate the effectiveness of the product.
14.1 Renal Tubular Acidosis (RTA) with Calcium Stones
The effect of oral potassium citrate therapy in a non-randomized, non-placebo controlled clinical study of five men and four women with calcium oxalate/calcium phosphate nephrolithiasis and documented incomplete distal renal tubular acidosis was examined. The main inclusion criterion was a history of stone passage or surgical removal of stones during the 3 years prior to initiation of potassium citrate therapy. All patients began alkali treatment with 60-80 mEq potassium citrate daily in 3 or 4 divided doses. Throughout treatment, patients were instructed to stay on a sodium restricted diet (100 mEq/day) and to reduce oxalate intake (limited intake of nuts, dark roughage, chocolate and tea). A moderate calcium restriction (400-800 mg/day) was imposed on patients with hypercalciuria.
X-rays of the urinary tract, available in all patients, were reviewed carefully to determine presence of pre-existing stones, appearance of new stones, or change in the number of stones.
Potassium citrate therapy was associated with inhibition of new stone formation in patients with distal tubular acidosis. Three of the nine patients continued to pass stones during the on-treatment phase. While it is likely that these patients passed pre-existing stones during therapy, the most conservative assumption is that the passed stones were newly formed. Using this assumption, the stone-passage remission rate was 67%. All patients had a reduced stone formation rate. Over the first 2 years of treatment, the on- treatment stone formation rate was reduced from 13±27 to 1±2 per year.
14.2 Hypocitraturic Calcium Oxalate Nephrolithiasis of any Etiology
Eighty-nine patients with hypocitraturic calcium nephrolithiasis or uric acid lithiasis with or without calcium nephrolithiasis participated in this non- randomized, non-placebo controlled clinical study. Four groups of patients were treated with potassium citrate: Group 1 was comprised of 19 patients, 10 with renal tubular acidosis and 9 with chronic diarrheal syndrome, Group 2 was comprised of 37 patients, 5 with uric acid stones alone, 6 with uric acid lithiasis and calcium stones, 3 with type 1 absorptive hypercalciuria, 9 with type 2 absorptive hypercalciuria and 14 with hypocitraturia. Group 3 was comprised of 15 patients with history of relapse on other therapy and Group 4 was comprised of 18 patients, 9 with type 1 absorptive hypercalciuria and calcium stones, 1 with type 2 absorptive hypercalciuria and calcium stones, 2 with hyperuricosuric calcium oxalate nephrolithiasis, 4 with uric acid lithiasis accompanied by calcium stones and 2 with hypocitraturia and hyperuricemia accompanied by calcium stones. The dose of potassium citrate ranged from 30 to 100 mEq per day, and usually was 20 mEq administered orally 3 times daily. Patients were followed in an outpatient setting every 4 months during treatment and were studied over a period from 1 to 4.33 years. A three- year retrospective pre-study history for stone passage or removal was obtained and corroborated by medical records. Concomitant therapy (with thiazide or allopurinol) was allowed if patients had hypercalciuria, hyperuricosuria or hyperuricemia. Group 2 was treated with potassium citrate alone.
In all groups, treatment that included potassium citrate was associated with a sustained increase in urinary citrate excretion from subnormal values to normal values (400 to 700 mg/day), and a sustained increase in urinary pH from 5.6-6.0 to approximately 6.5. The stone formation rate was reduced in all groups as shown inTable 1.
Table 1. Effect of Urocit-K In Patients With Calcium Oxalate Nephrolithiasis.
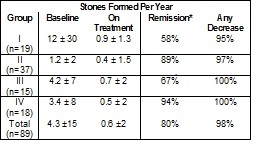
*Remission defined as “the percentage of patients remaining free of newly formed stones during treatment”.
14.3 Uric Acid Lithiasis with or without Calcium Stones
A long-term non-randomized, non-placebo controlled clinical trial with eighteen adult patients with uric acid lithiasis participated in the study. Six patients formed only uric acid stones, and the remaining 12 patients formed mixed stones containing both uric acid and calcium salts or formed both uric acid stones (without calcium salts) and calcium stones (without uric acid) on separate occasions.
Eleven of the 18 patients received potassium citrate alone. Six of the 7 other patients also received allopurinol for hyperuricemia with gouty arthritis, symptomatic hyperuricemia, or hyperuricosuria. One patient also received hydrochlorothiazide because of unclassified hypercalciuria. The main inclusion criterion was a history of stone passage or surgical removal of stones during the 3 years prior to initiation of potassium citrate therapy. All patients received potassium citrate at a dosage of 30-80 mEq/day in three-to-four divided doses and were followed every four months for up to 5 years.
While on potassium citrate treatment, urinary pH rose significantly from a low value of 5.3 ± 0.3 to within normal limits (6.2 to 6.5). Urinary citrate which was low before treatment rose to the high normal range and only one stone was formed in the entire group of 18 patients.
