ENALAPRIL MALEATE
ENALAPRIL MALEATE TABLETS, USP
a88902bd-742f-4ea6-b625-cc9df1709efc
HUMAN PRESCRIPTION DRUG LABEL
Sep 26, 2025
Amici Pharma, Inc
DUNS: 119294414
Products 4
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
enalapril maleate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
enalapril maleate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
enalapril maleate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
enalapril maleate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
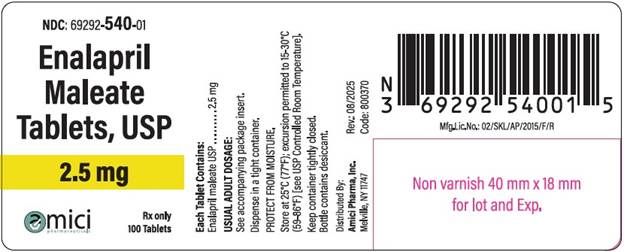
NDC: 69292-540-01
Enalapril Malete Tablets, USP
2.5 mg
Rx Only
100 Tablets
NDC: 69292-540-10
Enalapril Malete Tablets, USP
2.5 mg
Rx Only
1000 Tablets

NDC: 69292-542-01
Enalapril Malete Tablets, USP
5 mg
Rx Only
100 Tablets

NDC: 69292-542-10
Enalapril Malete Tablets, USP
5 mg
Rx Only
1000 Tablets

NDC: 69292-544-01
Enalapril Malete Tablets, USP
10 mg
Rx Only
100 Tablets

NDC: 69292-544-10
Enalapril Malete Tablets, USP
10 mg
Rx Only
1000 Tablets

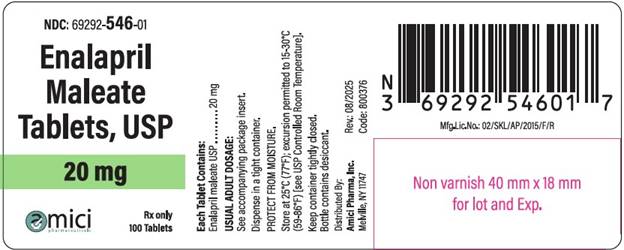
NDC: 69292-546-01
Enalapril Malete Tablets, USP
20 mg
Rx Only
100 Tablets
NDC: 69292-546-10
Enalapril Malete Tablets, USP
20 mg
Rx Only
1000 Tablets

