Baclofen
Baclofen
1b0f50c8-62e3-414d-96d3-5fd16e8043ab
HUMAN PRESCRIPTION DRUG LABEL
Sep 22, 2025
Marlex Pharmaceuticals Inc
DUNS: 782540215
Products 2
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
baclofen
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (5)
baclofen
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (5)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
NDC 10135-0533-10****
** Baclofen******
** Tablets, USP******
** 20 mg**
Rx Only
1000 TABLETS
DESCRIPTION SECTION
DESCRIPTION
Baclofen USP is a muscle relaxant and antispastic.
Its chemical name is 4-amino-3-(-4-chlorophenyl)-butanoic acid. The structural formula is:
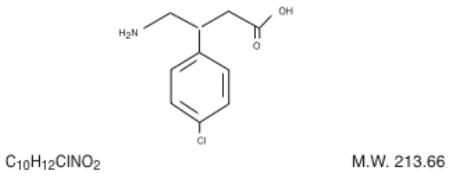
Baclofen USP is a white to off-white, odorless or practically odorless crystalline powder. It is slightly soluble in water, very slightly soluble in methanol and insoluble in chloroform.
Each tablet, for oral administration, contains 10 mg or 20 mg baclofen. In addition, each tablet contains the following inactive ingredients: microcrystalline cellulose, pregelatinized starch, colloidal silicon dioxide, and magnesium stearate.
