Buspirone hydrochloride
BUSPIRONE HYDROCHLORIDE TABLETS, USP (Patient Instruction Sheet Included) Rx only
3f66a3fe-9d73-180c-e063-6394a90aacca
HUMAN PRESCRIPTION DRUG LABEL
Sep 22, 2025
Radha Pharmaceuticals, Inc.
DUNS: 117634222
Products 5
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Buspirone hydrochloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
Buspirone hydrochloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
Buspirone hydrochloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
Buspirone hydrochloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
Buspirone hydrochloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL/PRINCIPAL DISPLAY PANEL
Buspirone Hydrochloride Tablets, USP 30 mg-Bottle of 60s
NDC 77771-238-60

INDICATIONS & USAGE SECTION
INDICATIONS AND USAGE
Buspirone hydrochloride tablets are indicated for the management of anxiety disorders or the short-term relief of the symptoms of anxiety. Anxiety or tension associated with the stress of everyday life usually does not require treatment with an anxiolytic.
The efficacy of buspirone hydrochloride tablets has been demonstrated in controlled clinical trials of outpatients whose diagnosis roughly corresponds to Generalized Anxiety Disorder (GAD). Many of the patients enrolled in these studies also had coexisting depressive symptoms and buspirone hydrochloride tablets relieved anxiety in the presence of these coexisting depressive symptoms. The patients evaluated in these studies had experienced symptoms for periods of 1 month to over 1 year prior to the study, with an average symptom duration of 6 months. Generalized Anxiety Disorder (300.02) is described in the American Psychiatric Association’s Diagnostic and Statistical Manual, III 1 as follows:
Generalized, persistent anxiety (of at least 1 month continual duration), manifested by symptoms from three of the four following categories:
- Motor tension: shakiness, jitteriness, jumpiness, trembling, tension, muscle aches, fatigability, inability to relax, eyelid twitch, furrowed brow, strained face, fidgeting, restlessness, easy startle.
- Autonomic hyperactivity: sweating, heart pounding or racing, cold, clammy hands, dry mouth, dizziness, lightheadedness, paresthesias (tingling in hands or feet), upset stomach, hot or cold spells, frequent urination, diarrhea, discomfort in the pit of the stomach, lump in the throat, flushing, pallor, high resting pulse, and respiration rate.
- Apprehensive expectation: anxiety, worry, fear, rumination, and anticipation of misfortune to self or others.
- Vigilance and scanning: hyperattentiveness resulting in distractibility, difficulty in concentrating, insomnia, feeling "on edge," irritability, impatience.
The above symptoms would not be due to another mental disorder, such as a depressive disorder or schizophrenia. However, mild depressive symptoms are common in GAD.
The effectiveness of buspirone hydrochloride tablets in long-term use, that is, for more than 3 to 4 weeks, has not been demonstrated in controlled trials. There is no body of evidence available that systematically addresses the appropriate duration of treatment for GAD. However, in a study of long- term use, 264 patients were treated with buspirone hydrochloride tablets for 1 year without ill effect. Therefore, the physician who elects to use buspirone hydrochloride tablets for extended periods should periodically reassess the usefulness of the drug for the individual patient.
CONTRAINDICATIONS SECTION
CONTRAINDICATIONS
Buspirone hydrochloride tablets are contraindicated in patients hypersensitive to buspirone hydrochloride.
The use of monoamine oxidase inhibitors (MAOIs) intended to treat depression with buspirone or within 14 days of stopping treatment with buspirone is contraindicated because of an increased risk of serotonin syndrome and/or elevated blood pressure. The use of buspirone within 14 days of stopping an MAOI intended to treat depression is also contraindicated.
Starting buspirone in a patient who is being treated with reversible MAOIs such as linezolid or intravenous methylene blue is also contraindicated because of an increased risk of serotonin syndrome. (seeWARNINGS, DOSAGE AND ADMINISTRATION and DRUG INTERACTIONS).
ADVERSE REACTIONS SECTION
Commonly Observed
The more commonly observed untoward events associated with the use of buspirone hydrochloride tablets not seen at an equivalent incidence among placebo-treated patients include dizziness, nausea, headache, nervousness, lightheadedness, and excitement.
DESCRIPTION SECTION
DESCRIPTION
Buspirone hydrochloride tablets, USP are an antianxiety agent that is not chemically or pharmacologically related to the benzodiazepines, barbiturates, or other sedative/anxiolytic drugs.
Buspirone hydrochloride, USP is a white crystalline, water soluble compound with a molecular weight of 421.96. Chemically, buspirone hydrochloride is 8-[4-[4-(2-pyrimidinyl)-1-piperazinyl]butyl] 8-azaspiro[4.5]decane-7,9-dione monohydrochloride. The empirical formula C 21H 31N 5O 2• HCl is represented by the following structural formula:

Buspirone hydrochloride is supplied as tablets for oral administration contains 5 mg, 7.5 mg, 10 mg, 15 mg or 30 mg of buspirone hydrochloride, USP (equivalent to 4.6 mg, 6.9 mg, 9.1 mg, 13.7 mg and 27.4 mg of buspirone free base, respectively). The 5 mg, 7.5 mg and 10 mg tablets are scored so they can be bisected. Thus, the 5 mg tablet can also provide a 2.5 mg dose, and the 10 mg tablet can provide a 5 mg dose. The 15 mg tablets are scored such that they may be bisected or trisected. Thus, a single tablet can provide the following doses: 15 mg (entire tablet), 10 mg (two-thirds of a tablet), 7.5 mg (one-half of a tablet) or 5 mg (one-third of a tablet). The 30 mg tablets are scored such that they may be bisected or trisected. Thus, a single tablet can provide the following doses: 30 mg (entire tablet), 20 mg (two-thirds of a tablet), 15 mg (one-half of a tablet), or 10 mg (one-third of a tablet). Buspirone hydrochloride tablets, USP contain the following inactive ingredients: colloidal silicon dioxide, lactose monohydrate, magnesium stearate, microcrystalline cellulose, and sodium starch glycolate.
CLINICAL PHARMACOLOGY SECTION
CLINICAL PHARMACOLOGY
The mechanism of action of buspirone is unknown. Buspirone differs from typical benzodiazepine anxiolytics in that it does not exert anticonvulsant or muscle relaxant effects. It also lacks the prominent sedative effect that is associated with more typical anxiolytics. In vitropreclinical studies have shown that buspirone has a high affinity for serotonin (5-HT 1A) receptors. Buspirone has no significant affinity for benzodiazepine receptors and does not affect GABA binding in vitroor in vivowhen tested in preclinical models.
Buspirone has moderate affinity for brain D 2-dopamine receptors. Some studies do suggest that buspirone may have indirect effects on other neurotransmitter systems.
Buspirone hydrochloride is rapidly absorbed in man and undergoes extensive first-pass metabolism. In a radiolabeled study, unchanged buspirone in the plasma accounted for only about 1% of the radioactivity in the plasma. Following oral administration, plasma concentrations of unchanged buspirone are very low and variable between subjects. Peak plasma levels of 1 ng/mL to 6 ng/mL have been observed 40 to 90 minutes after single oral doses of 20 mg. The single-dose bioavailability of unchanged buspirone when taken as a tablet is on the average about 90% of an equivalent dose of solution, but there is large variability.
The effects of food upon the bioavailability of buspirone hydrochloride tablets have been studied in eight subjects. They were given a 20 mg dose with and without food; the area under the plasma concentration-time curve (AUC) and peak plasma concentration (C max) of unchanged buspirone increased by 84% and 116%, respectively, but the total amount of buspirone immunoreactive material did not change. This suggests that food may decrease the extent of presystemic clearance of buspirone (seeDOSAGE AND ADMINISTRATION).
A multiple-dose study conducted in 15 subjects suggests that buspirone has nonlinear pharmacokinetics. Thus, dose increases and repeated dosing may lead to somewhat higher blood levels of unchanged buspirone than would be predicted from results of single-dose studies.
An in vitroprotein binding study indicated that approximately 86% of buspirone is bound to plasma proteins. It was also observed that aspirin increased the plasma levels of free buspirone by 23%, while flurazepam decreased the plasma levels of free buspirone by 20%. However, it is not known whether these drugs cause similar effects on plasma levels of free buspirone in vivo, or whether such changes, if they do occur, cause clinically significant differences in treatment outcome. An in vitrostudy indicated that buspirone did not displace highly protein-bound drugs such as phenytoin, warfarin, and propranolol from plasma protein, and that buspirone may displace digoxin.
Buspirone is metabolized primarily by oxidation, which in vitrohas been shown to be mediated by cytochrome P450 3A4 (CYP3A4) (SeePRECAUTIONS: Drug Interaction****s). Several hydroxylated derivatives and a pharmacologically active metabolite, 1-pyrimidinylpiperazine (1-PP), are produced. In animal models predictive of anxiolytic potential, 1-PP has about one quarter of the activity of buspirone, but is present in up to 20-fold greater amounts. However, this is probably not important in humans: blood samples from humans chronically exposed to buspirone hydrochloride tablets do not exhibit high levels of 1-PP; mean values are approximately 3 ng/mL and the highest human blood level recorded among 108 chronically dosed patients was 17 ng/mL, less than 1/200th of 1-PP levels found in animals given large doses of buspirone without signs of toxicity.
In a single-dose study using 14C-labeled buspirone, 29% to 63% of the dose was excreted in the urine within 24 hours, primarily as metabolites; fecal excretion accounted for 18% to 38% of the dose. The average elimination half- life of unchanged buspirone after single doses of 10 mg to 40 mg is about 2 to 3 hours.
Special Populations
Age and Gender Effects
After single or multiple doses in adults, no significant differences in buspirone pharmacokinetics (AUC and C max) were observed between elderly and younger subjects or between men and women.
Hepatic Impairment
After multiple-dose administration of buspirone to patients with hepatic impairment, steady-state AUC of buspirone increased 13-fold compared with healthy subjects (seePRECAUTIONS).
Renal Impairment
After multiple-dose administration of buspirone to renally impaired (Clcr = 10
to 70 mL/min/1.73 m 2) patients, steady-state AUC of buspirone increased
4-fold compared with healthy (Clcr ≥ 80 mL/min/1.73 m 2) subjects (see
PRECAUTIONS).
Race Effects
The effects of race on the pharmacokinetics of buspirone have not been
studied.
SPL UNCLASSIFIED SECTION
Central Nervous System
Frequent were dream disturbances; infrequent were depersonalization, dysphoria, noise intolerance, euphoria, akathisia, fearfulness, loss of interest, dissociative reaction, hallucinations, involuntary movements, slowed reaction time, suicidal ideation, and seizures; rare were feelings of claustrophobia, cold intolerance, stupor, and slurred speech and psychosis.
EENT
Frequent were tinnitus, sore throat, and nasal congestion; infrequent were
redness and itching of the eyes, altered taste, altered smell, and
conjunctivitis; rare were inner ear abnormality, eye pain, photophobia, and
pressure on eyes.
Endocrine
Rare were galactorrhea and thyroid abnormality.
Gastrointestinal
Infrequent were flatulence, anorexia, increased appetite, salivation,
irritable colon, and rectal bleeding; rare was burning of the tongue.
Genitourinary
Infrequent were urinary frequency, urinary hesitancy, menstrual irregularity
and spotting, and dysuria; rare were amenorrhea, pelvic inflammatory disease,
enuresis, and nocturia.
Musculoskeletal
Infrequent were muscle cramps, muscle spasms, rigid/stiff muscles, and
arthralgias; rare was muscle weakness.
Respiratory
Infrequent were hyperventilation, shortness of breath, and chest congestion;
rare was epistaxis.
Sexual Function
Infrequent were decreased or increased libido; rare were delayed ejaculation
and impotence.
Skin
Infrequent were edema, pruritus, flushing, easy bruising, hair loss, dry skin,
facial edema, and blisters; rare were acne and thinning of nails.
Clinical Laboratory
Infrequent were increases in hepatic aminotransferases (SGOT, SGPT); rare were
eosinophilia, leukopenia, and thrombocytopenia.
Miscellaneous
Infrequent were weight gain, fever, roaring sensation in the head, weight loss, and malaise; rare were alcohol abuse, bleeding disturbance, loss of voice, and hiccoughs.
Postmarketing Experience
Postmarketing experience has shown an adverse experience profile similar to
that given above. Voluntary reports since introduction have included rare
occurrences of allergic reactions (including urticaria), angioedema, cogwheel
rigidity, dizziness (rarely reported as vertigo), dystonic reactions
(including dystonia), ataxias, extrapyramidal symptoms, dyskinesias (acute and
tardive), ecchymosis, emotional lability, serotonin syndrome, transient
difficulty with recall, urinary retention, visual changes (including tunnel
vision), parkinsonism, akathisia, restless leg syndrome, and restlessness.
Because of the uncontrolled nature of these spontaneous reports, a causal
relationship to buspirone hydrochloride tablets treatment has not been
determined.
To report SUSPECTED ADVERSE REACTIONS, contact ScieGen Pharmaceutical, Inc. at 1-855-724-3436 or the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
WARNINGS SECTION
WARNINGS
**The administration of buspirone hydrochloride tablets to a patient taking a monoamine oxidase inhibitor (MAOI) may pose a hazard.**There have been reports of the occurrence of elevated blood pressure when buspirone hydrochloride tablets has been added to a regimen including an MAOI. Therefore, it is recommended that buspirone hydrochloride tablets not be used concomitantly with an MAOI.
PRECAUTIONS SECTION
PRECAUTIONS
General
Interference with Cognitive and Motor Performance
Studies indicate that buspirone hydrochloride tablets are less sedating than other anxiolytics and that it does not produce significant functional impairment. However, its CNS effects in any individual patient may not be predictable. Therefore, patients should be cautioned about operating an automobile or using complex machinery until they are reasonably certain that buspirone treatment does not affect them adversely.
While formal studies of the interaction of buspirone hydrochloride with alcohol indicate that buspirone does not increase alcohol-induced impairment in motor and mental performance, it is prudent to avoid concomitant use of alcohol and buspirone.
Potential for Withdrawal Reactions in Sedative/Hypnotic/Anxiolytic Drug- Dependent Patients
Because buspirone hydrochloride tablets do not exhibit cross-tolerance with benzodiazepines and other common sedative/hypnotic drugs, it will not block the withdrawal syndrome often seen with cessation of therapy with these drugs. Therefore, before starting therapy with buspirone hydrochloride tablets, it is advisable to withdraw patients gradually, especially patients who have been using a CNS-depressant drug chronically, from their prior treatment. Rebound or withdrawal symptoms may occur over varying time periods, depending in part on the type of drug, and its effective half-life of elimination.
The syndrome of withdrawal from sedative/hypnotic/anxiolytic drugs can appear as any combination of irritability, anxiety, agitation, insomnia, tremor, abdominal cramps, muscle cramps, vomiting, sweating, flu-like symptoms without fever, and occasionally, even as seizures.
Possible Concerns Related to Buspirone's Binding to Dopamine Receptors
Because buspirone can bind to central dopamine receptors, a question has been raised about its potential to cause acute and chronic changes in dopamine- mediated neurological function (e.g., dystonia, pseudo-parkinsonism, akathisia, and tardive dyskinesia). Clinical experience in controlled trials has failed to identify any significant neuroleptic-like activity; however, a syndrome of restlessness, appearing shortly after initiation of treatment, has been reported in some small fraction of buspirone-treated patients. The syndrome may be explained in several ways. For example, buspirone may increase central noradrenergic activity; alternatively, the effect may be attributable to dopaminergic effects (i.e., represent akathisia). SeeADVERSE REACTIONS: Postmarketing Experience.
DRUG ABUSE AND DEPENDENCE SECTION
DRUG ABUSE AND DEPENDENCE
Controlled Substance Class
Buspirone hydrochloride is not a controlled substance.
Physical and Psychological Dependence
In human and animal studies, buspirone has shown no potential for abuse or diversion and there is no evidence that it causes tolerance, or either physical or psychological dependence. Human volunteers with a history of recreational drug or alcohol usage were studied in two double-blind clinical investigations. None of the subjects were able to distinguish between buspirone hydrochloride tablets and placebo. By contrast, subjects showed a statistically significant preference for methaqualone and diazepam. Studies in monkeys, mice, and rats have indicated that buspirone lacks potential for abuse.
Following chronic administration in the rat, abrupt withdrawal of buspirone did not result in the loss of body weight commonly observed with substances that cause physical dependency.
Although there is no direct evidence that buspirone hydrochloride tablets causes physical dependence or drug-seeking behavior, it is difficult to predict from experiments the extent to which a CNS-active drug will be misused, diverted, and/or abused once marketed. Consequently, physicians should carefully evaluate patients for a history of drug abuse and follow such patients closely, observing them for signs of buspirone hydrochloride tablets misuse or abuse (e.g., development of tolerance, incrementation of dose, drug- seeking behavior).
OVERDOSAGE SECTION
OVERDOSAGE
Signs and Symptoms
In clinical pharmacology trials, doses as high as 375 mg/day were administered
to healthy male volunteers. As this dose was approached, the following
symptoms were observed: nausea, vomiting, dizziness, drowsiness, miosis, and
gastric distress. A few cases of overdosage have been reported, with complete
recovery as the usual outcome. No deaths have been reported following
overdosage with buspirone hydrochloride tablets alone. Rare cases of
intentional overdosage with a fatal outcome were invariably associated with
ingestion of multiple drugs and/or alcohol, and a causal relationship to
buspirone could not be determined. Toxicology studies of buspirone yielded the
following LD 50values: mice, 655 mg/kg; rats, 196 mg/kg; dogs, 586 mg/kg; and
monkeys, 356 mg/kg. These dosages are 160 to 550 times the recommended human
daily dose.
Recommended Overdose Treatment
General symptomatic and supportive measures should be used along with
immediate gastric lavage. Respiration, pulse, and blood pressure should be
monitored as in all cases of drug overdosage. No specific antidote is known to
buspirone, and dialyzability of buspirone has not been determined.
DOSAGE & ADMINISTRATION SECTION
DOSAGE AND ADMINISTRATION
The recommended initial dose is 15 mg daily (7.5 mg b.i.d.). To achieve an optimal therapeutic response, at intervals of 2 to 3 days the dosage may be increased 5 mg per day, as needed. The maximum daily dosage should not exceed 60 mg per day. In clinical trials allowing dose titration, divided doses of 20 mg to 30 mg per day were commonly employed.
The bioavailability of buspirone is increased when given with food as compared to the fasted state (seeCLINICAL PHARMACOLOGY). Consequently, patients should take buspirone in a consistent manner with regard to the timing of dosing; either always with or always without food.
When buspirone is to be given with a potent inhibitor of CYP3A4, the dosage recommendations described in thePRECAUTIONS: Drug Interactionssection should be followed.
Switching a Patient To or From a Monoamine Oxidase Inhibitor (MAOI) Antidepressant
At least 14 days should elapse between discontinuation of an MAOI intended to treat depression and initiation of therapy with buspirone hydrochloride tablets. Conversely, at least 14 days should be allowed after stopping buspirone hydrochloride tablets before starting an MAOI antidepressant (see CONTRAINDICATIONSandDRUG INTERACTIONS).
Use of Buspirone Hydrochloride Tablets with (Reversible) MAOIs, Such as Linezolid or Methylene Blue
Do not start buspirone hydrochloride tablets in a patient who is being treated with a reversible MAOI such as linezolid or intravenous methylene blue because there is an increased risk of serotonin syndrome. In a patient who requires more urgent treatment of a psychiatric condition, non-pharmacological interventions, including hospitalization, should be considered (see C****ONTRAINDICATIONSandDRUG INTERACTIONS).
In some cases, a patient already receiving therapy with buspirone hydrochloride tablets may require urgent treatment with linezolid or intravenous methylene blue. If acceptable alternatives to linezolid or intravenous methylene blue treatment are not available and the potential benefits of linezolid or intravenous methylene blue treatment are judged to outweigh the risks of serotonin syndrome in a particular patient, buspirone hydrochloride tablets should be stopped promptly, and linezolid or intravenous methylene blue can be administered. The patient should be monitored for symptoms of serotonin syndrome for 2 weeks or until 24 hours after the last dose of linezolid or intravenous methylene blue, whichever comes first. Therapy with buspirone hydrochloride tablets may be resumed 24 hours after the last dose of linezolid or intravenous methylene blue (seeWARNINGS).
The risk of administering methylene blue by non-intravenous routes (such as oral tablets or by local injection) or in intravenous doses much lower than 1 mg per kg with buspirone hydrochloride tablets is unclear. The clinician should, nevertheless, be aware of the possibility of emergent symptoms of serotonin syndrome with such use (see CONTRAINDICATIONS, WARNINGSand DRUG INTERACTIONS).
HOW SUPPLIED SECTION
HOW SUPPLIED
Buspirone Hydrochloride Tablets, USP 5 mg are available as white to slightly off-white, oval, uncoated tablets, debossed with “349” on one side and a score on the other side.
NDC 77771-234-01 Bottles of 100
NDC 77771-234-05 Bottles of 500
Buspirone Hydrochloride Tablets, USP 7.5 mg are available as white to slightly off-white, oval, uncoated tablets, debossed with “350” on one side and a score on the other side.
NDC 77771-235-01 Bottles of 100
Buspirone Hydrochloride Tablets, USP 10 mg are available as white to slightly off-white, oval, uncoated tablets, debossed with “351” on one side and a score on the other side.
NDC 77771-236-01 Bottles of 100
NDC 77771-236-05 Bottles of 500
Buspirone Hydrochloride Tablets, USP 15 mg are available as white to slightly off-white, rectangular, uncoated tablets, debossed with “3” and “52” with a score in between on one side and two scores on the other side. These tablets are scored to provide 15 mg (entire tablet), 10 mg (two-thirds of a tablet), 7.5 mg (one-half of a tablet) or 5 mg (one-third of a tablet).
NDC 77771-237-60 Bottles of 60
NDC 77771-237-05 Bottles of 500
Buspirone Hydrochloride Tablets, USP 30 mg are available as white to slightly off-white, rectangular, uncoated tablets, debossed with “3” and “53” with a score in between on one side and two scores on the other side. These tablets are scored to provide 30 mg (entire tablet), 20 mg (two-thirds of a tablet), 15 mg (one-half of a tablet) or 10 mg (one-third of a tablet).
NDC 77771-238-60 Bottles of 60
Store at 20° to 25° C (68° to 77° F) [see USP Controlled Room Temperature]. Dispense in a tight, light-resistant container as defined in the USP, with a child-resistant closure (as required).
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
REFERENCES SECTION
REFERENCE
1. American Psychiatric Association, Ed.: Diagnostic and Statistical Manual
of Mental Disorders-III, American Psychiatric Association, May 1980.
The brands listed are trademarks of their respective owners.
Manufactured by:
A&Z Pharmaceutical, Inc.
Hauppauge, NY 11788 USA
Distributed by:
Radha Pharmaceuticals, Inc.
Hauppauge, NY 11788 USA
Revised: 08/2025
PATIENT MEDICATION INFORMATION SECTION
PATIENT INSTRUCTION SHEET
Buspirone Hydrochloride (bue-SPYE-rone HYE-droe-KLOR-ide) Tablets, USP
Rx only
HOW TO USE BUSPIRONE HYDROCHLORIDE TABLETS, 15 mg
Response to buspirone varies among individuals. Your physician may find it
necessary to adjust your dosage to obtain the proper response.
This tablet design makes dosage adjustments easy. Each tablet is scored and
can be broken accurately to provide any of the following dosages:
If your doctor prescribed the 15 mg tablet:

To break a tablet accurately and easily, hold the tablet between your thumbs and index fingers close to the appropriate tablet score (groove) as shown below. Then, with the tablet score facing you, apply pressure and snap the tablet segments apart (segments breaking incorrectly should not be used).
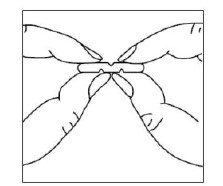
HOW TO USE BUSPIRONE HYDROCHLORIDE TABLETS, 30 mg
Response to buspirone varies among individuals. Your physician may find it
necessary to adjust your dosage to obtain the proper response.
This tablet design makes dosage adjustments easy. Each tablet is scored and
can be broken accurately to provide any of the following dosages:
If your doctor prescribed the 30 mg tablet:

To break a multi-scored tablet accurately and easily, hold the tablet between your thumbs and index fingers close to the appropriate tablet score (groove) as shown below.Then, with the tablet score facing you, apply pressure and snap the tablet segments apart (segments breaking incorrectly should not be used).
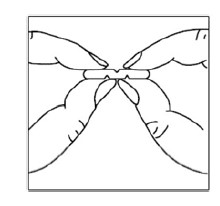
Manufactured by:
A&Z Pharmaceutical, Inc.
Hauppauge, NY 11788 USA
Distributed by:
Radha Pharmaceuticals, Inc.
Hauppauge, NY 11788 USA
Revised: 08/2025
LB8139
