Manufacturing Establishments2
FDA-registered manufacturing facilities and establishments involved in the production, packaging, or distribution of this drug product.
Bausch & Lomb Incorporated
079587625
Bausch & Lomb Incorporated
574139809
Products1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
LUMIFY PRESERVATIVE FREE EYE DROPS
Product Details
Drug Labeling Information
Complete FDA-approved labeling information including indications, dosage, warnings, contraindications, and other essential prescribing details.
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Package/Label Principal Display Panel- 20 ct Carton
BAUSCH + LOMB
NDC 24208-538-20
NEW
[Eye Image]
LUMIFY**®**
PRESERVATIVE FREE
BRIMONIDINE TARTRATE
OPHTHALMIC SOLUTION 0.025%
REDNESS RELIEVER EYE DROPS
*Now available in preservative free *Works in 1 minute *Convenient single-use vials
20 single-use vials (4 pouches x 5 single-use vials)
0.013 FL OZ (0.4 mL) per vial
[vial icon]
Sterile
622309
3912000
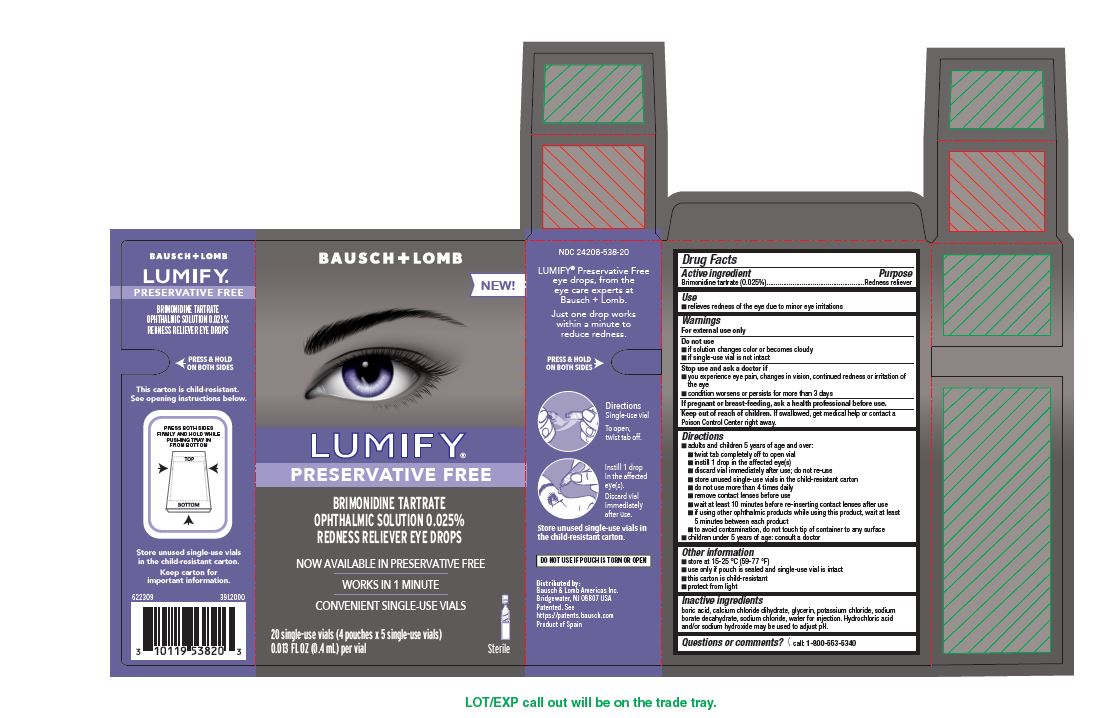
INDICATIONS & USAGE SECTION
Use
- relieves redness of the eye due to minor eye irritations
DOSAGE & ADMINISTRATION SECTION
Directions
- adults and children 5 years of age and over:
- twist tab completely off to open vial
- instill 1 drop in the affected eye(s)
- discard vial immediately after use; do not re-use
- store unused single-use vials in the child-resistant carton
- do not use more than 4 times daily
- remove contact lenses before use
- wait at least 10 minutes before re-inserting contact lenses after use
- if using other ophthalmic products while using this product, wait at least 5 minutes between each product
- to avoid contamination, do not touch tip of container to any surface
- children under 5 years of age: consult a doctor
WARNINGS SECTION
Warnings
For external use only
OTC - ACTIVE INGREDIENT SECTION
Active ingredient
Brimonidine tartrate (0.025%)
OTC - PURPOSE SECTION
Purpose
Redness reliever
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away
SPL UNCLASSIFIED SECTION
Questions or comments?[phone icon] call: 1-800-553-5340
INACTIVE INGREDIENT SECTION
Inactive Ingredients
boric acid, calcium chloride dihydrate, glycerin, potassium chloride, sodium borate decahydrate, sodium chloride, water for injection. Hydrochloric acid and/or sodium hydroxide may be used to adjust pH.
