Megace ES
These highlights do not include all the information needed to use Megace ES safely and effectively. See full prescribing information for Megace ES. Megace ES (megestrol acetate, USP) Oral Suspension Initial U.S. Approval: 1993
654854bc-f847-4e3b-a0a1-8716553460ac
HUMAN PRESCRIPTION DRUG LABEL
Mar 18, 2014
Atlantic Biologicals Corps
DUNS: 047437707
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
megesterol acetate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (10)
Drug Labeling Information
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
The efficacy of Megace ES (megestrol acetate oral suspension, 125 mg/mL) was based on two trials of megestrol acetate oral suspension (40 mg/mL). These two trials are described below. ®
Trial 1
One was a multicenter, randomized, double-blind, placebo-controlled study comparing megestrol acetate (MA) at doses of 100 mg, 400 mg, and 800 mg per day versus placebo in AIDS patients with anorexia/cachexia and significant weight loss. Of the 270 patients entered on study, 195 met all inclusion/exclusion criteria, had at least two additional post baseline weight measurements over a 12 week period or had one post baseline weight measurement but dropped out for therapeutic failure. The percent of patients gaining 2.3 kg or more at maximum weight gain in 12 study weeks was statistically significantly greater for the 800 mg (64%) and 400 mg (57%) MA-treated groups than for the placebo group (24%). Mean weight increased from baseline to last evaluation in 12 study weeks in the 800 mg MA-treated group by 3.5 kg, the 400 mg MA group by 1.9 kg, the 100 mg MA group by 0.9 kg and decreased in the placebo group by 0.7 kg. Mean weight changes at 4, 8 and 12 weeks for patients evaluable for efficacy in the two clinical trials is shown graphically. Changes in body composition during the 12 study weeks as measured by bioelectrical impedance analysis showed increases in non-water body weight in the MA-treated groups. In addition, edema developed or worsened in only 3 patients.
Greater percentages of MA-treated patients in the 800 mg group (89%), the 400 mg group (68%) and the 100 mg group (72%), than in the placebo group (50%), showed an improvement in appetite at last evaluation during the 12 study weeks. A statistically significant difference was observed between the 800 mg MA-treated group and the placebo group in the change in caloric intake from baseline to time of maximum weight change. Patients were asked to assess weight change, appetite, appearance, and overall perception of well-being in a 9 question survey. At maximum weight change only the 800 mg MA-treated group gave responses that were statistically significantly more favorable to all questions when compared to the placebo-treated group. A dose response was noted in the survey with positive responses correlating with higher dose for all questions.
Trial 2
The second trial was a multicenter, randomized, double-blind, placebo-controlled study comparing megestrol acetate 800 mg/day versus placebo in AIDS patients with anorexia/cachexia and significant weight loss. Of the 100 patients entered on study, 65 met all inclusion/exclusion criteria, had at least two additional post baseline weight measurements over a 12 week period or had one post baseline weight measurement but dropped out for therapeutic failure. Patients in the 800 mg MA-treated group had a statistically significantly larger increase in mean maximum weight change than patients in the placebo group. From baseline to study week 12, mean weight increased by 5.1 kg in the MA-treated group and decreased 1.0 kg in the placebo group. Changes in body composition as measured by bioelectrical impedance analysis showed increases in non-water weight in the MA-treated group (see Clinical Studies table). No edema was reported in the MA-treated group. A greater percentage of MA-treated patients (67%) than placebo-treated patients (38%) showed an improvement in appetite at last evaluation during the 12 study weeks; this difference was statistically significant. There were no statistically significant differences between treatment groups in mean caloric change or in daily caloric intake at time to maximum weight change. In the same 9 question survey referenced in the first trial, patients' assessments of weight change, appetite, appearance, and overall perception of well-being showed increases in mean scores in MA-treated patients as compared to the placebo group.
In both trials, patients tolerated the drug well and no statistically significant differences were seen between the treatment groups with regard to laboratory abnormalities, new opportunistic infections, lymphocyte counts, T4 counts, T8 counts, or skin reactivity tests [see Adverse Reactions (6)].
|
Megestrol Acetate Oral Suspension Clinical Efficacy Trials | ||||||
|
Trial 1 Study Accrual Dates 11/88 to 12/90 |
Trial 2 Study Accrual Dates 5/89 to 4/91 | |||||
|
Megestrol Acetate, mg/day |
0 |
100 |
400 |
800 |
0 |
800 |
|
38 |
82 |
75 |
75 |
48 |
52 |
|
28 |
61 |
53 |
53 |
29 |
36 |
|
Mean Change in Weight (kg) | ||||||
|
0.0 |
1.3 |
4.2 |
4.9 |
-1.0 |
5.1 |
|
21 |
44 |
57 |
64 |
28 |
47 |
| ||||||
|
0.0 |
1.0 |
1.3 |
2.5 |
0.7 |
2.6 |
|
-0.8 |
-0.1 |
0.7 |
1.1 |
-0.7 |
-0.3 |
|
-1.3 |
-0.3 |
0.0 |
0.0 |
-0.1 |
-0.1 |
| ||||||
| ||||||
|
50 |
72 |
72 |
93 |
48 |
69 |
|
50 |
72 |
68 |
89 |
38 |
67 |
| ||||||
| ||||||
|
-107 |
326 |
308 |
646 |
30 |
464 |
|
*Based on bioelectrical impedance analysis determinations at last evaluation in 12 weeks. |
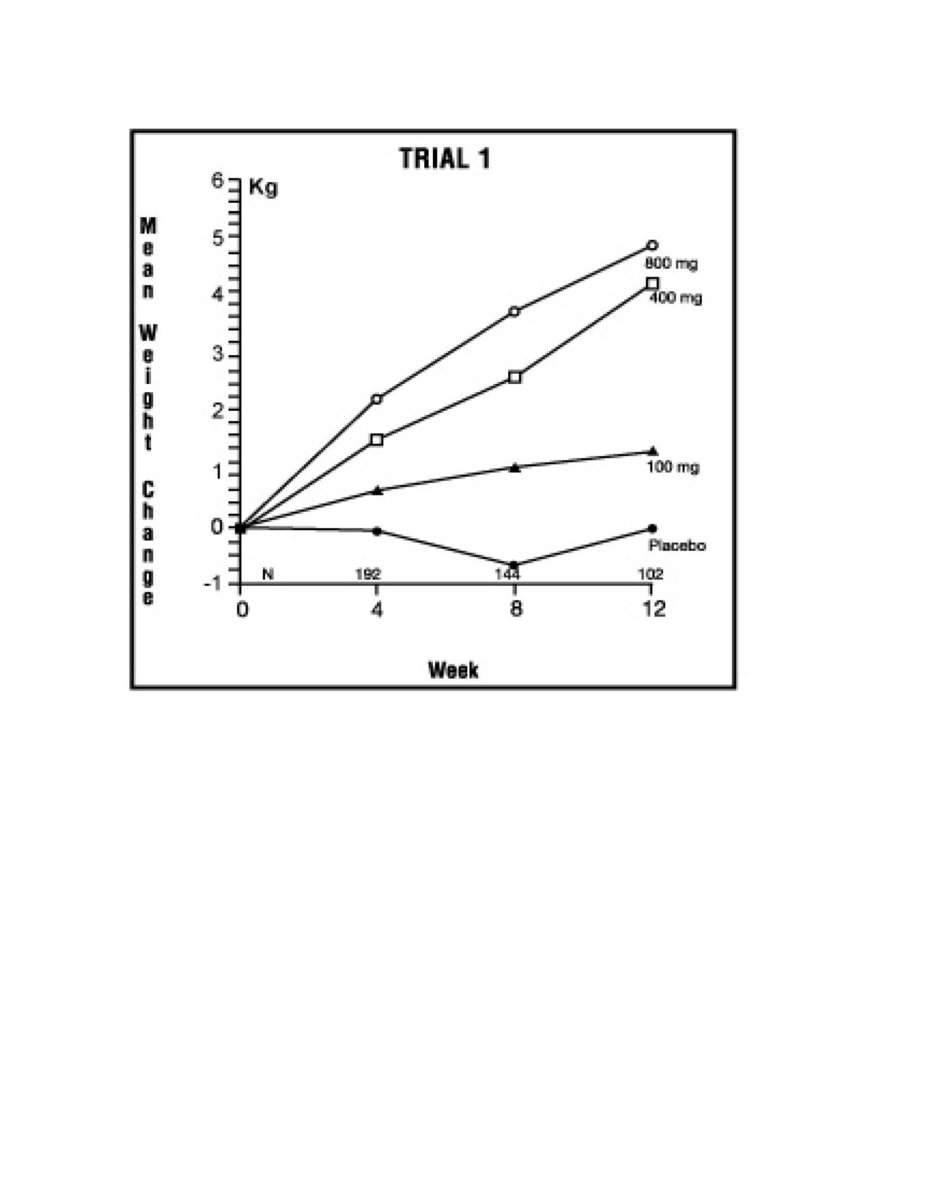
Figure 2: Mean Weight Change for Patients Evaluable for Efficacy in Trial 1
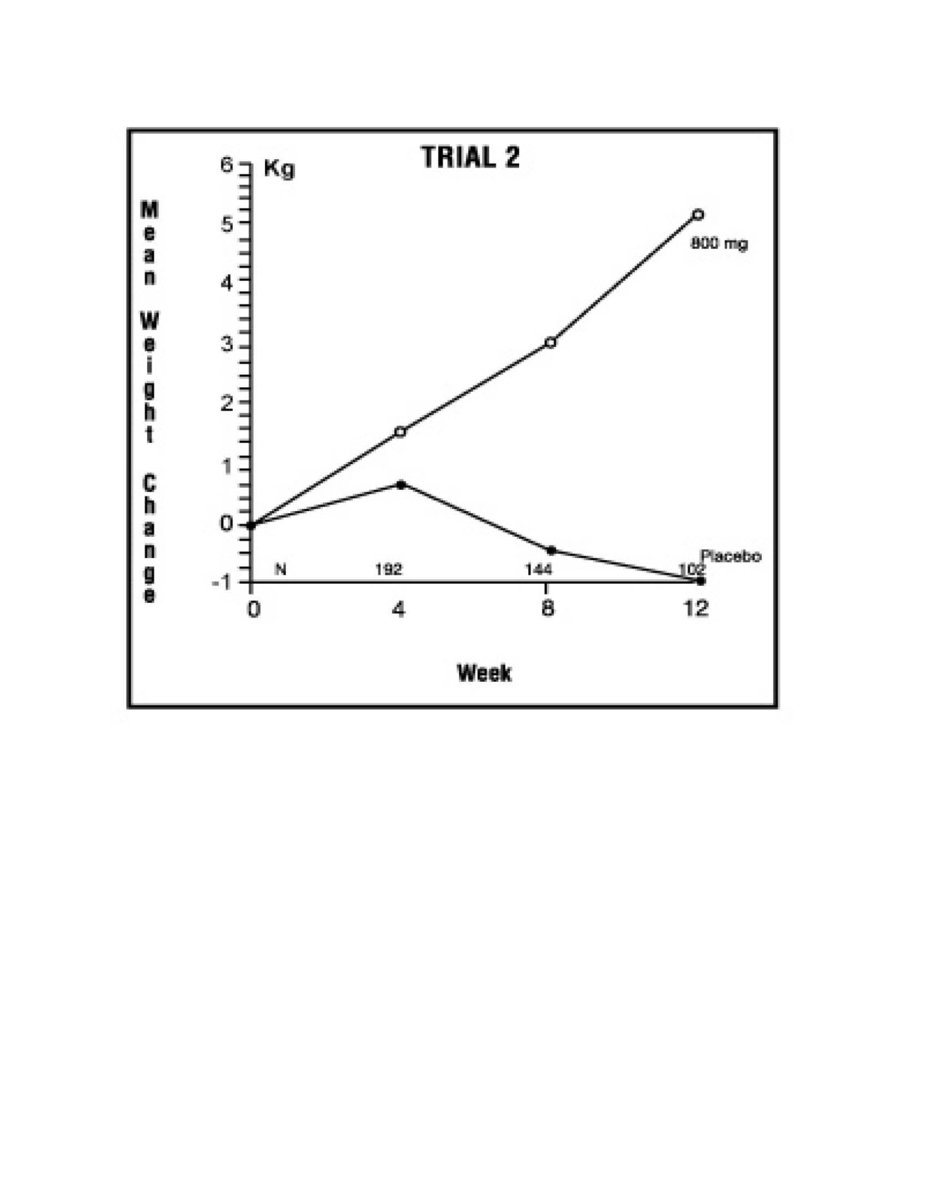
Figure 3: Mean Weight Change for Patients Evaluable for Efficacy in Trial 2
