Vapor Inhaler
Drug Facts
69059828-25dc-444f-bab4-0ec5325e5b91
HUMAN OTC DRUG LABEL
Sep 19, 2025
Geiss, Destin & Dunn, Inc.
DUNS: 076059836
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Levmetamfetamine
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
Drug Labeling Information
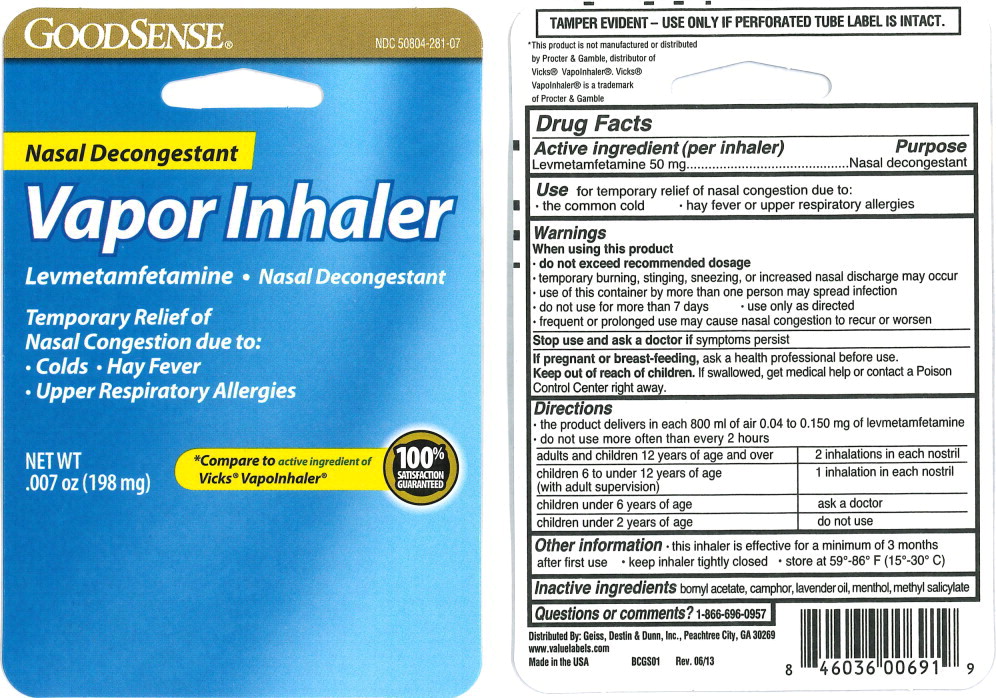
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Principal Display Panel - 198 mg Blister Pack Label
GOODSENSE**®**
NDC 50804-281-07
Nasal Decongestant
Vapor Inhaler
Levmetamfetamine • Nasal Decongestant
Temporary Relief of
Nasal Congestion due to:
*Colds • Hay Fever *Upper Respiratory Allergies
NET WT
.007 oz (198 mg)
Compare to active ingredient of
Vicks®* Vapolnhaler****®**
100%
SATISFACTION
GUARANTEED
INDICATIONS & USAGE SECTION
Use
for temporary relief of nasal congestion due to:
- the common cold
- hay fever or upper respiratory allergies
OTC - ACTIVE INGREDIENT SECTION
Active ingredient (per inhaler)
Levmetamfetamine 50 mg
OTC - PURPOSE SECTION
Purpose
Nasal decongestant
INACTIVE INGREDIENT SECTION
Inactive ingredients
bornyl acetate, camphor, lavender oil, menthol, methyl salicylate
OTC - QUESTIONS SECTION
Questions or comments?
1-866-696-0957
WARNINGS SECTION
Warnings
When using this product
*do not exceed recommended dosage
-
temporary burning, stinging, sneezing, or increased nasal discharge may occur
-
use of this container by more than one person may spread infection
-
do not use for more than 7 days
-
use only as directed
-
frequent or prolonged use may cause nasal congestion to recur or worsen
Stop use and ask a doctor if symptoms persist
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
DOSAGE & ADMINISTRATION SECTION
Directions
- the product delivers in each 800 ml of air 0.04 to 0.150 mg of levmetamfetamine
- do not use more often than every 2 hours
|
adults and children 12 years of age and over |
2 inhalations in each nostril |
|
children 6 to under 12 years of age (with adult supervision) |
1 inhalation in each nostril |
|
children under 6 years of age |
ask a doctor |
|
children under 2 years of age |
do not use |
OTHER SAFETY INFORMATION
Other information
- this inhaler is effective for a minimum of 3 months after first use
- keep inhaler tightly closed
- store at 59°-86° F (15°-30° C)
