ENTECAVIR
These highlights do not include all the information needed to use ENTECAVIR TABLETS safely and effectively. See full prescribing information for ENTECAVIR TABLETS. ENTECAVIR tablets, for oral use Initial U.S. Approval: 2005
da705339-c349-444f-a058-4f0cef2864bf
HUMAN PRESCRIPTION DRUG LABEL
Nov 24, 2022
NorthStar RxLLC
DUNS: 830546433
Products 2
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
ENTECAVIR
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (10)
ENTECAVIR
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (9)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
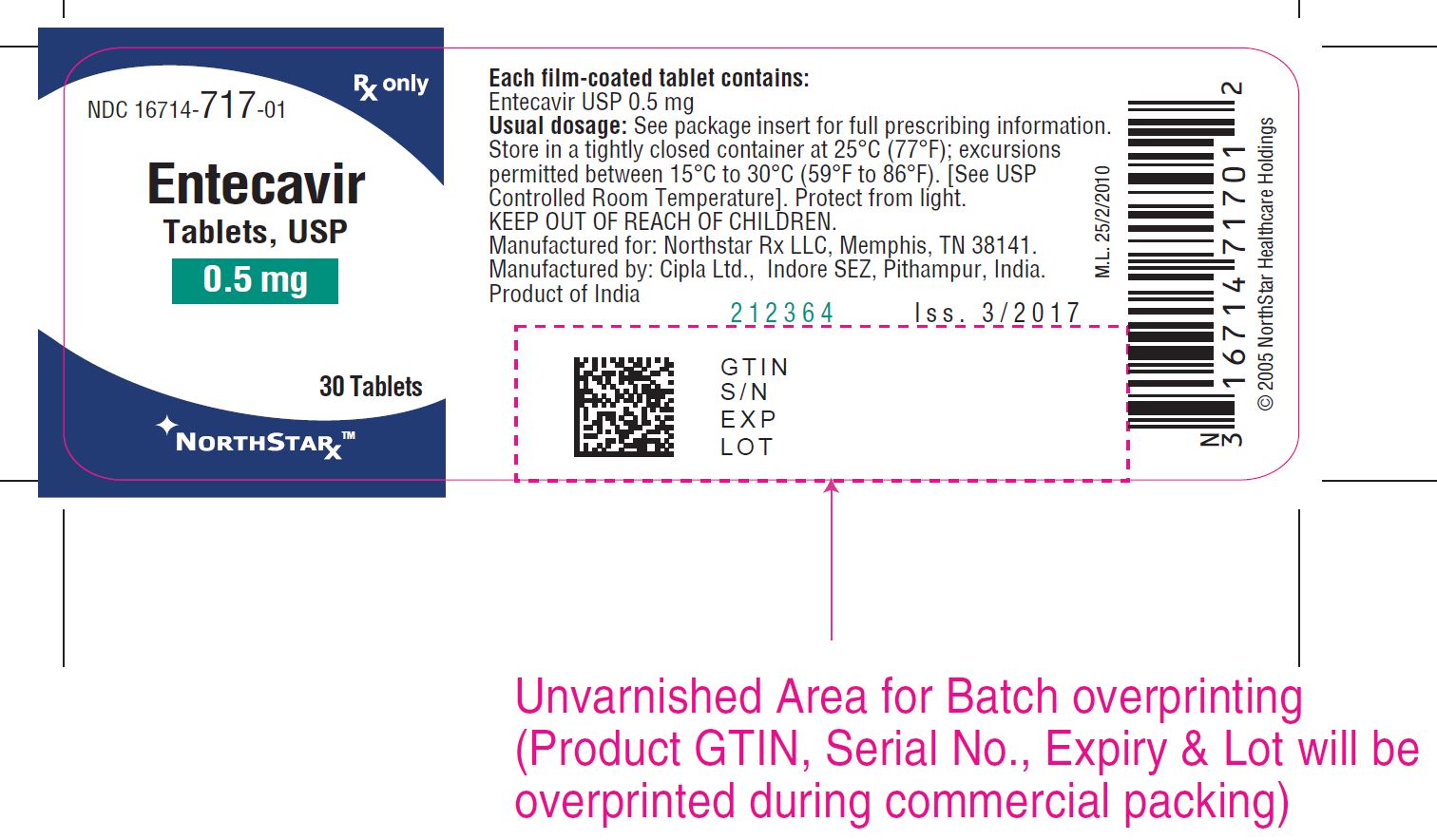
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC 16714-717-01 RX only
Entecavir
Tablets, USP
** 0.5 mg**
30 Tablets
NORTHSTAR
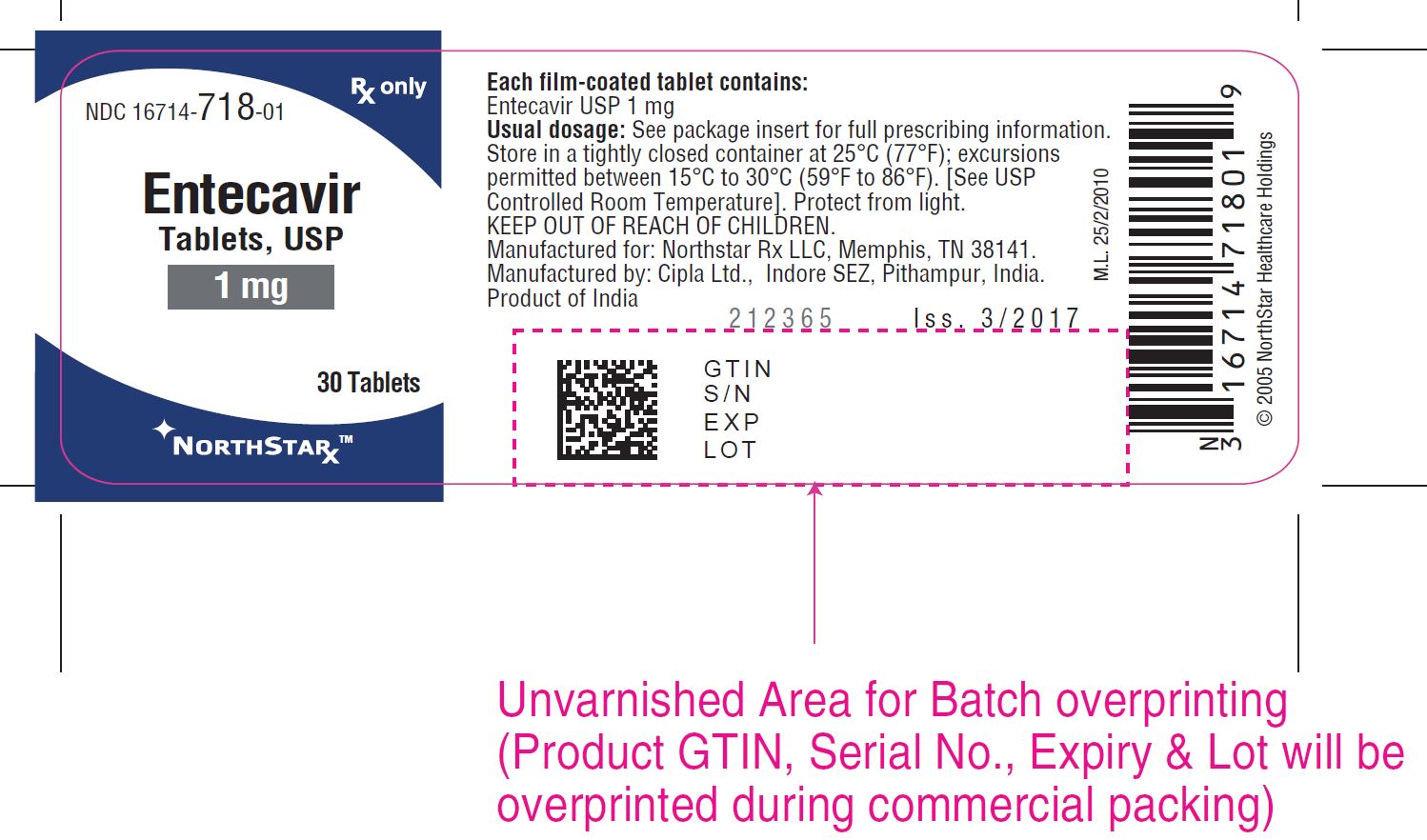
NDC 16714-718-01 RX only
Entecavir
Tablets, USP
** 1 mg**
30 Tablets
NORTHSTAR