Bortezomib
These highlights do not include all the information needed to use BORTEZOMIB FOR INJECTION safely and effectively. See full prescribing information for BORTEZOMIB FOR INJECTION. BORTEZOMIB for injection, for subcutaneous or intravenous use Initial U.S. Approval: 2003
191d231a-1fbd-4a65-9d33-d5dc03bac381
HUMAN PRESCRIPTION DRUG LABEL
May 2, 2022
Pharmascience Inc.
DUNS: 202657094
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
bortezomib
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (2)
Drug Labeling Information
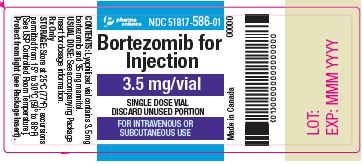
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
INTRAVENOUS INJECTION ONLY
0.9% NaCl
Add 3.5 mL
0.9% Sodium Chloride
To make 1 mg / mL final concentration
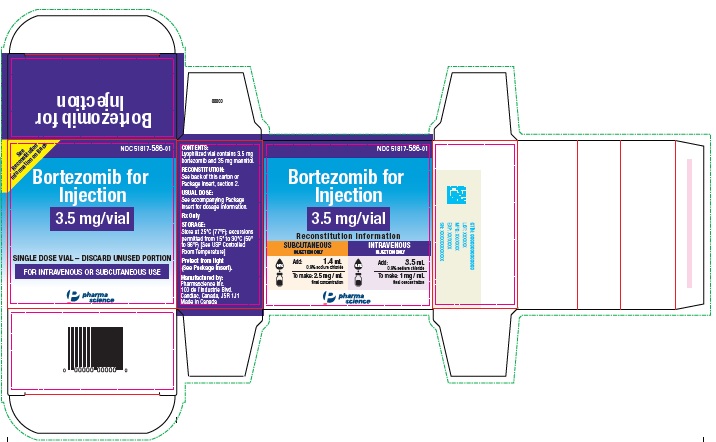
PRINCIPAL DISPLAY PANEL - 3.5 mg Vial Label
Bortezomib for Injection
3.5 mg/vial
SINGLE DOSE VIAL
DISCARD UNUSED PORTION
FOR INTRAVENOUS OR
SUBCUTANEOUS USE
INDICATIONS & USAGE SECTION
1 INDICATIONS AND USAGE
1.1 Multiple Myeloma
Bortezomib for injection , 3.5 mg/vial is indicated for the treatment of adult patients with multiple myeloma.
1.2 Mantle Cell Lymphoma
Bortezomib for injection is indicated for the treatment of adult patients with mantle cell lymphoma.
Bortezomib for injection is a proteasome inhibitor indicated for:
- treatment of adult patients with multiple myeloma ( 1.1)
- treatment of adult patients with mantle cell lymphoma ( 1.2)
CONTRAINDICATIONS SECTION
4 CONTRAINDICATIONS
Bortezomib for injection is contraindicated in patients with hypersensitivity (not including local reactions) to bortezomib, boron, or mannitol. Reactions have included anaphylactic reactions [ see Adverse Reactions (6.1)].
Bortezomib for injection is contraindicated for intrathecal administration. Fatal events have occurred with intrathecal administration of Bortezomib for injection.
- Patients with hypersensitivity (not including local reactions) to bortezomib, boron, or mannitol, including anaphylactic reactions. ( 4)
- Contraindicated for intrathecal administration. ( 4)
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
7.1 Effects of Other Drugs on Bortezomib for injection
Strong CYP3A4 Inducers Coadministration with a strong CYP3A4 inducer decreases the exposure of bortezomib [ see Clinical Pharmacology (12.3)] which may decrease Bortezomib for injection efficacy. Avoid coadministration with strong CYP3A4 inducers.
Strong CYP3A4 Inhibitors Coadministration with a strong CYP3A4 inhibitor increases the exposure of bortezomib [ see Clinical Pharmacology (12.3)] which may increase the risk of Bortezomib for injection toxicities. Monitor patients for signs of bortezomib toxicity and consider a bortezomib dose reduction if bortezomib must be given in combination with strong CYP3A4 inhibitors.
7.2 Drugs without Clinically Significant Interactions with Bortezomib for
injection
No clinically significant drug interactions have been observed when Bortezomib for injection was coadministered with dexamethasone, omeprazole, or melphalan in combination with prednisone [ see Clinical Pharmacology (12.3)].
- Strong CYP3A4 Inhibitors: Closely monitor patients with concomitant use. ( 7.1)
- Strong CYP3A4 Inducers: Avoid concomitant use. (7.3)
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosing Guidelines
Bortezomib for injection is for intravenous or subcutaneous use only. Do not administer Bortezomib for injection by any other route.
Because each route of administration has a different reconstituted concentration, use caution when calculating the volume to be administered.
The recommended starting dose of Bortezomib for injection is 1.3 mg/m 2. Bortezomib for injection is administered intravenously at a concentration of 1 mg/mL, or subcutaneously at a concentration of 2.5 mg/mL [ see Dosage and Administration (2.10)].
Bortezomib for injection retreatment may be considered for patients with multiple myeloma who had previously responded to treatment with Bortezomib for injection and who have relapsed at least six months after completing prior Bortezomib for injection treatment. Treatment may be started at the last tolerated dose [ see Dosage and Administration (2.6)]
When administered intravenously, administer Bortezomib for injection as a 3 to 5 second bolus intravenous injection.
2.2 Dosage in Previously Untreated Multiple Myeloma
Bortezomib for injection is administered in combination with oral melphalan and oral prednisone for 9, six-week treatment cycles as shown in Table 1. In Cycles 1 to 4, Bortezomib for injection is administered twice weekly (Days 1, 4, 8, 11, 22, 25, 29 and 32). In Cycles 5 to 9, Bortezomib for injection is administered once weekly (Days 1, 8, 22 and 29). At least 72 hours should elapse between consecutive doses of Bortezomib for injection .
Table 1: Dosage Regimen for Patients with Previously Untreated Multiple Myeloma|
** Twice Weekly Bortezomib for injection (Cycles 1 to 4)** | ||||||||||||
|
Week |
1 |
2 |
3 |
4 |
5 |
6 | ||||||
|
Bortezomib for injection |
Day |
-- |
-- |
Day |
Day |
Day |
rest |
Day |
Day |
Day |
Day |
rest |
|
Melphalan(9 mg/m 2) |
Day |
Day |
Day |
Day |
-- |
-- |
rest |
-- |
-- |
-- |
-- |
rest |
|
** Once Weekly Bortezomib for injection (Cycles 5 to 9 when used in combination with Melphalan and Prednisone)** | ||||||||||||
|
** Week** |
** 1** |
** 2** |
** 3** |
** 4** |
** 5** |
** 6** | ||||||
|
Bortezomib for injection |
Day |
-- |
-- |
Day |
rest |
Day |
Day |
rest | ||||
|
Melphalan(9 mg/m 2) |
Day |
Day |
Day |
Day |
-- |
-- |
rest |
-- |
-- |
-- |
-- |
rest |
2.3 Dose Modification Guidelines for BORTEZOMIB FOR INJECTION When Given in
Combination with Melphalan and Prednisone
Prior to initiating any cycle of therapy with Bortezomib for injection in combination with melphalan and prednisone:
- Platelet count should be at least 70 x 10 9/L and the absolute neutrophil count (ANC) should be at least 1 x 10 9/L
- Nonhematological toxicities should have resolved to Grade 1 or baseline
|
Toxicity |
Dose Modification or Delay |
|---|---|
|
Hematological toxicity during a cycle: |
Consider reduction of the melphalan dose by 25% in the next cycle |
|
If platelet count is not above 30 x 10 9/L or ANC is not above 0.75 x 10 9/L on a Bortezomib for injection dosing day (other than Day 1) |
Withhold Bortezomib for injection dose |
|
If several Bortezomib for injection doses in consecutive cycles are withheld due to toxicity |
Reduce Bortezomib for injection dose by one dose level (from 1.3 mg/m 2 to 1 mg/m 2, or from 1 mg/m 2 to 0.7 mg/m 2) |
|
Grade 3 or higher nonhematological toxicities |
Withhold Bortezomib for injection therapy until symptoms of toxicity have resolved to Grade 1 or baseline. Then, Bortezomib for injection may be reinitiated with one dose level reduction (from 1.3 mg/m 2 to 1 mg/m 2, or from 1 mg/m 2 to 0.7 mg/m 2). For Bortezomib for injection-related neuropathic pain and/or peripheral neuropathy, hold or modify Bortezomib for injection as outlined in Table 5. |
For information concerning melphalan and prednisone, see manufacturer's prescribing information.
Dose modifications guidelines for peripheral neuropathy are provided [ see Dosage and Administration (2.7)]
2.4 Dosage in Previously Untreated Mantle Cell Lymphoma
Bortezomib for injection (1.3 mg/m 2) is administered intravenously in combination with intravenous rituximab, cyclophosphamide, doxorubicin and oral prednisone (VcR-CAP) for 6, three week treatment cycles as shown in Table 3. Bortezomib for injection is administered first followed by rituximab. Bortezomib for injection is administered twice weekly for two weeks (Days 1, 4, 8, and 11) followed by a ten day rest period on Days 12 to 21. For patients with a response first documented at Cycle 6, two additional VcR-CAP cycles are recommended. At least 72 hours should elapse between consecutive doses of Bortezomib for injection.
Table: 3 Dosage Regime for Patients with Previously Untreated Mantle Cell Lymphoma Twice Weekly Bortezomib for injection (6, Three Week Cycles)*
| ||||||||
|
Week |
1 |
2 |
3 | |||||
|
Bortezomib for Injection (1.3 mg/m 2) |
Day 1 |
-- |
-- |
Day 4 |
-- |
Day 8 |
Day 11 |
rest period |
|
Rituximab (375 mg/m 2) |
Day 1 |
-- |
-- |
-- |
-- |
rest period | ||
|
Prednisone (100 mg/m 2) |
Day 1 |
Day 2 |
Day 3 |
Day 4 |
Day 5 |
-- |
-- |
rest period |
2.5 Dose Modification Guidelines for Bortezomib for injection When Given in
Combination with Rituximab, Cyclophosphamide, Doxorubicin and Prednisone
Prior to the first day of each cycle (other than Cycle 1):
- Platelet count should be at least 100 x 10 9/L and absolute neutrophil count (ANC) should be at least 1.5 x 10 9/L
- Hemoglobin should be at least 8 g/dL (at least 4.96 mmol/L)
- Nonhematologic toxicity should have recovered to Grade 1 or baseline
Interrupt Bortezomib for injection treatment at the onset of any Grade 3 hematologic or nonhematological toxicities, excluding neuropathy [ see Table 5, Warnings and Precautions (5)]. For dose adjustments, see Table 4 below.
Table 4: Dose Modifications on Days 4, 8, and 11 During Cycles of Combination Bortezomib for Injection, Rituximab, Cyclophosphamide, Doxorubicin and Prednisone Therapy|
Toxicity |
Dose Modification or Delay |
|
Hematological Toxicity | |
|
• Grade 3 or higher neutropenia, or a platelet count not at or above 25 x 10 9/L |
• Withhold Bortezomib for injection therapy for up to 2 weeks until patient
has an ANC at or above 0.75 x 10 9/L and a platelet count at or above 25 x 10
9/L. •If toxicity resolves such that the patient has an ANC at or above 0.75 x 10 9/L and a platelet count at or above 25 x 10 9/L, Bortezomib for injection dose should be reduced by 1 dose level (from 1.3 mg/m 2 to 1 mg/m 2, or from 1 mg/m 2 to 0.7 mg/m 2) |
|
Grade 3 or higher nonhematological toxicities |
Withhold Bortezomib for Injection therapy until symptoms of the toxicity have resolved to Grade 2 or better. Then, Bortezomib for injection may be reinitiated with one dose level reduction (from 1.3 mg/m 2, or from 1 mg/m 2 to 0.7 mg/m 2). For Bortezomib Injection-related neuropathic pain and/or peripheral neuropathy, hold or modify Bortezomib for injection as outlined in Table 5. |
For information concerning rituximab, cyclophosphamide, doxorubicin and prednisone, see manufacturer's prescribing information.
2.6 Dosage and Dose Modifications for Relapsed Multiple Myeloma and
Relapsed Mantle Cell Lymphoma
Bortezomib for injection (1.3 mg/m 2/dose) is administered twice weekly for two weeks (Days 1, 4, 8, and 11) followed by a ten day rest period (Days 12 to 21). For extended therapy of more than eight cycles, Bortezomib for injection may be administered on the standard schedule or, for relapsed multiple myeloma, on a maintenance schedule of once weekly for four weeks (Days 1, 8, 15, and 22) followed by a 13 day rest period (Days 23 to 35) [ see Clinical Studies (14)]. At least 72 hours should elapse between consecutive doses of Bortezomib for injection.
Patients with multiple myeloma who have previously responded to treatment with Bortezomib for injection (either alone or in combination) and who have relapsed at least six months after their prior Bortezomib for injection therapy may be started on Bortezomib for injection at the last tolerated dose. Retreated patients are administered Bortezomib for injection twice weekly (Days 1, 4, 8, and 11) every three weeks for a maximum of eight cycles. At least 72 hours should elapse between consecutive doses of Bortezomib for injection. Bortezomib for injection may be administered either as a single agent or in combination with dexamethasone [ see Clinical Studies (14.1)].
Bortezomib for injection therapy should be withheld at the onset of any Grade 3 nonhematological or Grade 4 hematological toxicities excluding neuropathy as discussed below [ see Warnings and Precautions (5)]. Once the symptoms of the toxicity have resolved, Bortezomib for injection therapy may be reinitiated at a 25% reduced dose (1.3 mg/m 2/dose reduced to 1 mg/m 2/dose; 1 mg/m 2/dose reduced to 0.7 mg/m 2/dose).
For dose modifications guidelines for peripheral neuropathy see section 2.7.
2.7 Dose Modifications for Peripheral Neuropathy
Starting Bortezomib for injection subcutaneously may be considered for patients with pre-existing or at high risk of peripheral neuropathy. Patients with pre-existing severe neuropathy should be treated with Bortezomib for injection only after careful risk-benefit assessment.
Patients experiencing new or worsening peripheral neuropathy during Bortezomib for injection therapy may require a decrease in the dose and/or a less dose- intense schedule.
For dose or schedule modification guidelines for patients who experience Bortezomib for injection-related neuropathic pain and/or peripheral neuropathy see Table 5.
Table 5: Recommended Dose Modification for Bortezomib for injection- Related Neuropathic Pain and/or Peripheral Sensory or Motor Neuropathy|
Severity of Peripheral Neuropathy Signs and Symptoms * |
Modification of Dose and Regimen |
|---|---|
|
Grade 1 (asymptomatic; loss of deep tendon reflexes or paresthesia) without pain or loss of function |
No action |
|
Grade 1 with pain or Grade 2 (moderate symptoms; limiting instrumental Activities of Daily Living (ADL)**) |
Reduce Bortezomib for injection to 1 mg/m 2 |
|
Grade 2 with pain or Grade 3 (severe symptoms; limiting self care ADL***) |
Withhold Bortezomib for injection therapy until toxicity resolves. When toxicity resolves reinitiate with a reduced dose of Bortezomib for injection at 0.7 mg/m 2 once per week. |
|
Grade 4 (life-threatening consequences; urgent intervention indicated) |
Discontinue Bortezomib for injection |
*Grading based on NCI Common Terminology Criteria CTCAE v4.0
** Instrumental ADL: refers to preparing meals, shopping for groceries or
clothes, using telephone, managing money, etc.
*** Self care ADL: refers to bathing, dressing and undressing, feeding self,
using the toilet, taking medications, and not bedridden
2.8 Dosage in Patients with Hepatic Impairment
Do not adjust the starting dose for patients with mild hepatic impairment.
Start patients with moderate or severe hepatic impairment started on Bortezomib for injection dose at a reduced dose of 0.7 mg/m 2 per injection during the first cycle, and consider subsequent dose escalation to 1 mg/m 2 or further dose reduction to 0.5 mg/m 2 based on patient tolerance (see Table 6) [ see Use in Specific Populations (8.7), and Clinical Pharmacology (12.3)].
Table 6: Recommended Starting Dose Modification for Bortezomib for injection in Patients with Hepatic Impairment|
Bilirubin Level |
SGOT (AST) Levels |
Modification of Starting Dose | |
|---|---|---|---|
|
Abbreviations: SGOT = serum glutamic oxaloacetic transaminase; | |||
|
Mild |
Less than or equal to 1x ULN |
More than ULN |
None |
|
More than 1x to 1.5x ULN |
Any |
None | |
|
Moderate |
More than 1.5x to 3x ULN |
Any |
Reduce Bortezomib for injection to 0.7 mg/m 2 in the first cycle. Consider dose escalation to 1 mg/m 2 or further dose reduction to 0.5 mg/m 2 in subsequent cycles based on patient tolerability. |
|
Severe |
More than 3x ULN |
Any |
2.9 Administration Precautions
The drug quantity contained in one vial (3.5 mg) may exceed the usual dose required. Caution should be used in calculating the dose to prevent overdose [ see Dosage and Administration (2.10)] .
When administered subcutaneously, sites for each injection (thigh or abdomen) should be rotated. New injections should be given at least one inch from an old site and never into areas where the site is tender, bruised, erythematous, or indurated.
If local injection site reactions occur following Bortezomib for injection administration subcutaneously, a less concentrated Bortezomib for injection solution (1 mg/mL instead of 2.5 mg/mL) may be administered subcutaneously [ see Dosage and Administration (2.10)]. Alternatively, consider use of the intravenous route of administration [ see Dosage and Administration (2.10)].
Bortezomib for injection is a hazardous drug. Follow applicable special handling and disposal procedure. 1
2.10 Reconstitution/Preparation for Intravenous and Subcutaneous
Administration
Use proper aseptic technique. Reconstitute only with 0.9% sodium chloride. The reconstituted product should be a clear and colorless solution.
Different volumes of 0.9% sodium chloride are used to reconstitute the product for the different routes of administration. The reconstituted concentration of bortezomib for subcutaneous administration (2.5 mg/mL) is greater than the reconstituted concentration of bortezomib for intravenous administration (1 mg/mL).Because each route of administration has a different reconstituted concentration, use caution when calculating the volume to be administered[ see Dosage and Administration (2.9)].
For each 3.5 mg single-dose vial of bortezomib reconstitute with the following volume of 0.9% sodium chloride based on route of administration ( Table 7):
Table 7: Reconstitution Volumes and Final Concentration for Intravenous and Subcutaneous Administration|
Route of Administration |
Bortezomib |
Diluent |
Final Bortezomib concentration (mg/mL) |
|---|---|---|---|
|
Intravenous |
3.5 mg |
3.5 mL |
1 mg/mL |
|
Subcutaneous |
3.5 mg |
1.4 mL |
2.5 mg/mL |
Dose must be individualized to prevent overdosage. After determining patient body surface area (BSA) in square meters, use the following equations to calculate the total volume (mL) of reconstituted Bortezomib for injection to be administered:
*Intravenous Administration [1 mg/mL concentration]
Bortezomib for injection dose (mg/m 2) x patient BSA (m 2) = Total Bortezomib
for injection volume (mL) to be administered
1 mg/mL
*Subcutaneous Administration [2.5 mg/mL concentration]
Bortezomib for injection dose (mg/m 2)x patient BSA (m 2) = Total Bortezomib
for injection volume (mL) to be administered
2.25 mg/mL
Stickers that indicate the route of administration are provided with each Bortezomib for injection vial. These stickers should be placed directly on the syringe of Bortezomib for injection once Bortezomib for injection is prepared to help alert practitioners of the correct route of administration for Bortezomib for injection.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit. If any discoloration or particulate matter is observed, the reconstituted product should not be used.
Stability:
Unopened vials of Bortezomib for injection are stable until the date indicated
on the package when stored in the original package protected from light.
Bortezomib for injection contains no antimicrobial preservative. Administer reconstituted Bortezomib for injection within eight hours of preparation. When reconstituted as directed, Bortezomib for injection may be stored at 25°C (77°F). The reconstituted material may be stored in the original vial and/or the syringe prior to administration. The product may be stored for up to eight hours in a syringe; however, total storage time for the reconstituted material must not exceed eight hours when exposed to normal indoor lighting.
-
For subcutaneous or intravenous use only. Each route of administration has a different reconstituted concentration; Exercise caution when calculating the volume to be administered. ( 2.1, 2.10)
-
The recommended starting dose of Bortezomib for injection is 1.3 mg/m 2 administered either as a 3 to 5 second bolus intravenous injection or subcutaneous injection. ( 2.2, 2.4, 2.6)
-
Retreatment for Multiple Myeloma: May retreat starting at the last tolerated dose. ( 2.6)
-
Hepatic Impairment: Use a lower starting dose for patients with moderate or severe hepatic impairment. ( 2.8)
-
Dose must be individualized to prevent overdose ( 2.10)
DOSAGE FORMS & STRENGTHS SECTION
3 DOSAGE FORMS AND STRENGTHS
For injection: Each single-dose vial of Bortezomib for injection contains 3.5 mg of bortezomib as a sterile lyophilized white to off-white powder for reconstitution and withdrawal of the appropriate individual patient dose [ see Dosage and Administration (2.10)].
- For injection: Single-dose vial contains 3.5 mg of bortezomib as lyophilized powder for reconstitution and withdrawal of the appropriate individual patient dose. ( 3)
USE IN SPECIFIC POPULATIONS SECTION
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on its mechanism of action [ see Clinical Pharmacology (12.1) ] and findings in animals, Bortezomib for injection can cause fetal harm when administered to a pregnant woman. There are no studies with the use of Bortezomib for injection in pregnant women to inform drug-associated risks. Bortezomib caused embryo-fetal lethality in rabbits at doses lower than the clinical dose ( see Data). Advise pregnant women of the potential risk to the fetus
Adverse outcomes in pregnancy occur regardless of the health of the mother or the use of medications. The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Animal Data
Bortezomib was not teratogenic in nonclinical developmental toxicity studies in rats and rabbits at the highest dose tested (0.075 mg/kg; 0.5 mg/m 2 in the rat and 0.05 mg/kg; 0.6 mg/m 2 in the rabbit) when administered during organogenesis. These dosages are approximately 0.5 times the clinical dose of 1.3 mg/m 2 based on body surface area.
Bortezomib caused embryo-fetal lethality in rabbits at doses lower than the clinical dose (approximately 0.5 times the clinical dose of 1.3 mg/m2 based on body surface area). Pregnant rabbits given bortezomib during organogenesis at a dose of 0.05 mg/kg (0.6 mg/m 2) experienced significant postimplantation loss and decreased number of live fetuses. Live fetuses from these litters also showed significant decreases in fetal weight.
8.2 Lactation
Risk Summary
There are no data on the presence of bortezomib or its metabolites in human
milk, the effects of the drug on the breastfed child, or the effects of the
drug on milk production. Because many drugs are excreted in human milk and
because the potential for serious adverse reactions in a breastfed child from
Bortezomib for injection is unknown, advise nursing women not to breastfeed
during treatment with Bortezomib for injection and for two months after
treatment.
8.3 Females and Males of Reproductive Potential
Based on its mechanism of action and findings in animals, Bortezomib for
injection can cause fetal harm when administered to a pregnant woman [ see Use in Specific Populations (8.1)].
Pregnancy Testing
Conduct pregnancy testing in females of reproductive potential prior to
initiating Bortezomib for injection treatment.
Contraception
Females
Advise females of reproductive potential to use effective contraception during
treatment with Bortezomib for injection and for seven months after the last
dose.
Males
Males with female partners of reproductive potential should use effective
contraception during treatment with Bortezomib for injection and for four
months after the last dose.
Infertility
Based on the mechanism of action and findings in animals, Bortezomib for
injection may have an effect on either male or female fertility [ see Nonclinical Toxicology (13.1)].
8.4 Pediatric Use
Safety and effectiveness have not been established in pediatric patients.
The activity and safety of Bortezomib for injection in combination with intensive reinduction chemotherapy was evaluated in pediatric and young adult patients with lymphoid malignancies (pre-B cell ALL 77%, 16% with T-cell ALL, and 7% T-cell lymphoblastic lymphoma (LL)), all of whom relapsed within 36 months of initial diagnosis in a single-arm multicenter, nonrandomized cooperative group trial. An effective reinduction multiagent chemotherapy regimen was administered in three blocks. Block 1 included vincristine, prednisone, doxorubicin and pegaspargase; Block 2 included cyclophosphamide, etoposide and methotrexate; Block 3 included high-dose cytosine arabinoside and asparaginase. Bortezomib for injection was administered at a dose of 1.3 mg/m2 as a bolus intravenous injection on Days 1, 4, 8, and 11 of Block 1 and Days 1, 4, and 8 of Block 2. There were 140 patients with ALL or LL enrolled and evaluated for safety. The median age was ten years (range: 1 to 26), 57% were male, 70% were white, 14% were black, 4% were Asian, 2% were American Indian/ Alaska Native, 1% were Pacific Islander.
The activity was evaluated in a prespecified subset of the first 60 evaluable patients enrolled on the study with pre-B ALL ≤21 years and relapsed <36 months from diagnosis. The complete remission (CR) rate at day 36 was compared to that in a historical control set of patients who had received the identical backbone therapy without Bortezomib for injection There was no evidence that the addition of Bortezomib for injection had any impact on the CR rate.
No new safety concerns were observed when Bortezomib for injection was added to a chemotherapy backbone regimen as compared with a historical control group in which the backbone regimen was given without Bortezomib for injection.
The BSA-normalized clearance of bortezomib in pediatric patients was similar to that observed in adults.
8.5 Geriatric Use
Of the 669 patients enrolled in the relapsed multiple myeloma study, 245 (37%) were 65 years of age or older: 125 (38%) on the Bortezomib for injection arm and 120 (36%) on the dexamethasone arm. Median time to progression and median duration of response for patients ≥65 were longer on Bortezomib for injection compared to dexamethasone [5.5 mo vs 4.3 mo, and 8.0 mo vs 4.9 mo, respectively]. On the Bortezomib for injection arm, 40% (n=46) of evaluable patients aged ≥65 experienced response (CR + PR) vs 18% (n=21) on the dexamethasone arm. The incidence of Grade 3 and 4 events was 64%, 78% and 75% for Bortezomib for injection patients ≤50, 51-64 and ≥65 years old, respectively [ see Adverse Reactions (6.1), Clinical Studies (14.1)].
No overall differences in safety or effectiveness were observed between patients ≥age 65 and younger patients receiving Bortezomib for injection; but greater sensitivity of some older individuals cannot be ruled out.
8.6 Renal Impairment
No starting dosage adjustment of Bortezomib for injection is recommended for patients with renal impairment. In patients requiring dialysis, Bortezomib for injection should be administered after the dialysis procedure [ see Clinical Pharmacology (12.3)].
8.7 Hepatic Impairment
No starting dosage adjustment of Bortezomib for injection is recommended for patients with mild hepatic impairment (total bilirubin ≤1x ULN and AST >ULN, or total bilirubin >1 to 1.5x ULN and any AST). The exposure of bortezomib is increased in patients with moderate (total bilirubin ≥1.5 to 3x ULN and any AST) and severe (total bilirubin >3x ULN and any AST) hepatic impairment. Reduce the starting dose in patients with moderate or severe hepatic impairment [ see Dosage and Administration (2.8), Clinical Pharmacology (12.3)].
8.8 Patients with Diabetes
During clinical trials, hypoglycemia and hyperglycemia were reported in diabetic patients receiving oral hypoglycemics. Patients on oral antidiabetic agents receiving Bortezomib for injection treatment may require close monitoring of their blood glucose levels and adjustment of the dose of their antidiabetic medication.
Patients with diabetes may require close monitoring of blood glucose and adjustment of antidiabetic medication. ( 8.8)
OVERDOSAGE SECTION
10 OVERDOSAGE
There is no known specific antidote for Bortezomib for injection overdosage. In humans, fatal outcomes following the administration of more than twice the recommended therapeutic dose have been reported, which were associated with the acute onset of symptomatic hypotension (5.2) and thrombocytopenia (5.7). In the event of an overdosage, the patient's vital signs should be monitored and appropriate supportive care given.
Studies in monkeys and dogs showed that intravenous bortezomib doses as low as two times the recommended clinical dose on a mg/m 2 basis were associated with increases in heart rate, decreases in contractility, hypotension, and death. In dog studies, a slight increase in the corrected QT interval was observed at doses resulting in death. In monkeys, doses of 3.0 mg/m 2 and greater (approximately twice the recommended clinical dose) resulted in hypotension starting at one hour postadministration, with progression to death in 12 to 14 hours following drug administration.
DESCRIPTION SECTION
11 DESCRIPTION
Bortezomib for injection, 3.5 mg/vial a proteasome inhibitor, contains bortezomib which is an antineoplastic agent. Bortezomib is a modified dipeptidylboronic acid. The chemical name for bortezomib, the monomeric boronic acid, is [(1R)-3-methyl-1-[[(2S)-1-oxo-3-phenyl-2-[(pyrazinylcarbonyl) amino]propyl]amino]butyl] boronic acid.
Bortezomib has the following chemical structure:

The molecular weight is 384.24. The molecular formula is C 19H 25BN 4O 4. The solubility of bortezomib, as the monomeric boronic acid, in water is 3.3 to 3.8 mg/mL in a pH range of 2 to 6.5.
Bortezomib for injection, 3.5 mg/vial is available for intravenous injection or subcutaneous use. Each single-dose vial contains 3.5 mg of bortezomib as a sterile lyophilized powder. It also contains the inactive ingredient: 35 mg mannitol, USP. The product is provided as a mannitol boronic ester which, in reconstituted form, consists of the mannitol ester in equilibrium with its hydrolysis product, the monomeric boronic acid. The drug substance exists in its cyclic anhydride form as a trimeric boroxine.
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Bortezomib is a reversible inhibitor of the chymotrypsin-like activity of the 26S proteasome in mammalian cells. The 26S proteasome is a large protein complex that degrades ubiquitinated proteins. The ubiquitin-proteasome pathway plays an essential role in regulating the intracellular concentration of specific proteins, thereby maintaining homeostasis within cells. Inhibition of the 26S proteasome prevents this targeted proteolysis, which can affect multiple signaling cascades within the cell. This disruption of normal homeostatic mechanisms can lead to cell death. Experiments have demonstrated that bortezomib is cytotoxic to a variety of cancer cell types in vitro. Bortezomib causes a delay in tumor growth in vivo in nonclinical tumor models, including multiple myeloma.
12.2 Pharmacodynamics
Following twice weekly administration of 1 mg/m 2 and 1.3 mg/m 2 bortezomib doses, the maximum inhibition of 20S proteasome activity (relative to baseline) in whole blood was observed five minutes after drug administration. Comparable maximum inhibition of 20S proteasome activity was observed between 1 and 1.3 mg/m 2 doses. Maximal inhibition ranged from 70% to 84% and from 73% to 83% for the 1 mg/m 2 and 1.3 mg/m 2 dose regimens, respectively.
12.3 Pharmacokinetics
Following intravenous administration of 1 mg/m 2 and 1.3 mg/m 2 doses, the mean maximum plasma concentrations of bortezomib (C max) after the first dose (Day 1) were 57 and 112 ng/mL, respectively. When administered twice weekly, the mean maximum observed plasma concentrations ranged from 67 to 106 ng/mL for the 1 mg/m 2 dose and 89 to 120 ng/mL for the 1.3 mg/m 2 dose.
Following an intravenous bolus or subcutaneous injection of a 1.3 mg/m 2 dose to patients with multiple myeloma, the total systemic exposure after repeat dose administration (AUC last) was equivalent for subcutaneous and intravenous administration. The AUC last geometric mean ratio (90% confidence interval) was 0.99 (0.80 to 1.23). The C max after subcutaneous administration (20.4 ng/mL) was lower than after intravenous administration (223 ng/mL) with repeat dose administration.
Distribution
The mean distribution volume of bortezomib ranged from approximately 498 to
1884 L/m 2 following single- or repeat-dose administration of 1 mg/m 2 or
1.3mg/m 2 to patients with multiple myeloma. The binding of bortezomib to
human plasma proteins averaged 83% over the concentration range of 100 to 1000
ng/mL.
Elimination
The mean elimination half-life of bortezomib upon multiple dosing ranged from
40 to 193 hours after the 1 mg/m2 dose and 76 to 108 hours after the 1.3 mg/m
2 dose. The mean total body clearances were 102 and 112 L/h following the
first dose for doses of 1 mg/m 2 and 1.3 mg/m 2, respectively, and ranged from
15 to 32 L/h following subsequent doses for doses of 1 and 1.3 mg/m 2,
respectively.
Metabolism
Bortezomib is primarily oxidatively metabolized to several inactive
metabolites in vitro via cytochrome P450 (CYP) enzymes 3A4, CYP2C19, and
CYP1A2, and to a lesser extent by CYP2D6 and CYP2C9.
Excretion
The pathways of elimination of bortezomib have not been characterized in
humans.
Specific Populations
No clinically significant differences in the pharmacokinetics of bortezomib
were observed based on age, sex, or renal impairment (including patients
administered Bortezomib for injection after dialysis). The effect of race on
bortezomib pharmacokinetics is unknown.
Patients with Hepatic Impairment
Following administration of bortezomib doses ranging from 0.5 to 1.3 mg/m 2,
mild (total bilirubin ≤1x ULN and AST >ULN, or total bilirubin >1 to 1.5x ULN
and any AST) hepatic impairment did not alter dose-normalized bortezomib AUC
when compared to patients with normal hepatic function. Dose normalized mean
bortezomib AUC increased by approximately 60% in patients with moderate (total
bilirubin >1.5 to 3x ULN and any AST) or severe hepatic impairment. A lower
starting dose is recommended in patients with moderate or severe (total
bilirubin >3x ULN and any AST) hepatic hepatic impairment.
Drug Interaction Studies
Clinical Studies
No clinically significant differences in bortezomib pharmacokinetics were
observed when coadministered with dexamethasone (weak CYP3A4 inducer),
omeprazole (strong CYP2C19 inhibitor), or melphalan in combination with
prednisone.
Strong CYP3A4 Inhibitor
Coadministration with ketoconazole (strong CYP3A4 inhibitor) increased
bortezomib exposure by 35%.
Strong CYP3A4 Inducer
Coadministration with rifampin (strong CYP3A4 inducer) decreased bortezomib
exposure by approximately 45%.
In Vitro Studies
Bortezomib may inhibit CYP2C19 activity and increase exposure to drugs that
are substrates for this enzyme.
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies have not been conducted with bortezomib.
Bortezomib showed clastogenic activity (structural chromosomal aberrations) in the in vitro chromosomal aberration assay using Chinese hamster ovary cells. Bortezomib was not genotoxic when tested in the in vitro mutagenicity assay (Ames test) and in vivo micronucleus assay in mice.
Fertility studies with bortezomib were not performed but evaluation of reproductive tissues has been performed in the general toxicity studies. In the six month rat toxicity study, degenerative effects in the ovary were observed at doses ≥ 0.3 mg/m 2 (one-fourth of the recommended clinical dose), and degenerative changes in the testes occurred at 1.2 mg/m 2.
13.2 Animal Toxicology and/or Pharmacology
Cardiovascular Toxicity
Studies in monkeys showed that administration of dosages approximately twice
the recommended clinical dose resulted in heart rate elevations, followed by
profound progressive hypotension, bradycardia, and death 12 to 14 hours
postdose. Doses ≥ 1.2 mg/m 2 induced dose-proportional changes in cardiac
parameters. Bortezomib has been shown to distribute to most tissues in the
body, including the myocardium. In a repeated dosing toxicity study in the
monkey, myocardial hemorrhage, inflammation, and necrosis were also observed.
Chronic Administration
In animal studies at a dose and schedule similar to that recommended for
patients (twice weekly dosing for two weeks followed by one week rest),
toxicities observed included severe anemia and thrombocytopenia, and
gastrointestinal, neurological and lymphoid system toxicities. Neurotoxic
effects of bortezomib in animal studies included axonal swelling and
degeneration in peripheral nerves, dorsal spinal roots, and tracts of the
spinal cord. Additionally, multifocal hemorrhage and necrosis in the brain,
eye, and heart were observed.
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
14.1 Multiple Myeloma
Randomized, Open-Label Clinical Study in Patients with Previously Untreated Multiple Myeloma
A prospective, international, randomized (1:1), open-label clinical study of 682 (NCT00111319) patients was conducted to determine whether Bortezomib for injection administered intravenously (1.3 mg/m 2) in combination with melphalan (9 mg/m 2) and prednisone (60 mg/m 2) resulted in improvement in time to progression (TTP) when compared to melphalan (9 mg/m 2) and prednisone (60 mg/m 2) in patients with previously untreated multiple myeloma. Treatment was administered for a maximum of nine cycles (approximately 54 weeks) and was discontinued early for disease progression or unacceptable toxicity. Antiviral prophylaxis was recommended for patients on the Bortezomib for injection study arm.
The median age of the patients in the study was 71 years (48;91), 50% were male, 88% were Caucasian and the median Karnofsky performance status score for the patients was 80 (60;100). Patients had IgG/IgA/Light chain myeloma in 63%/25%/8% instances, a median hemoglobin of 105 g/L (64;165), and a median platelet count of 221,500 /microliter (33,000;587,000).
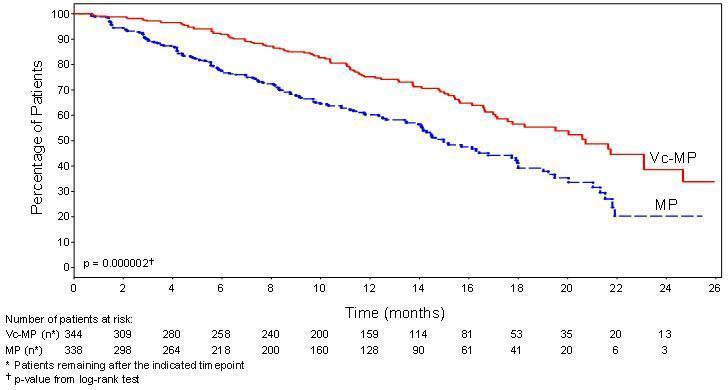
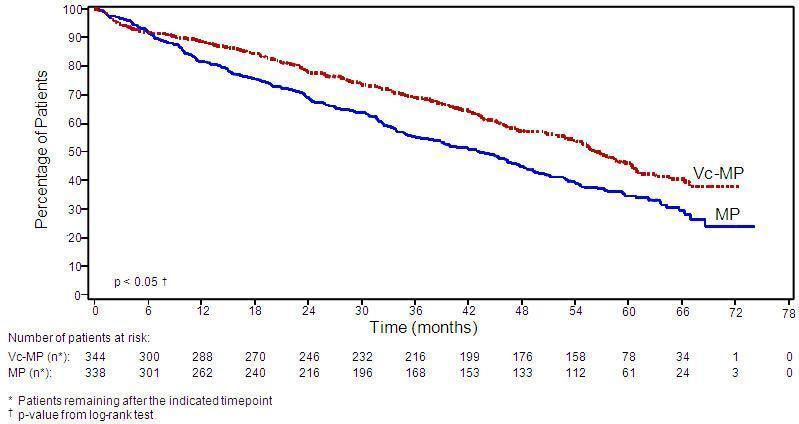
Efficacy results for the trial are presented in Table 14. At a prespecified interim analysis (with median follow-up of 16.3 months), the combination of Bortezomib for injection, melphalan and prednisone therapy resulted in significantly superior results for time to progression, progression-free survival, overall survival and response rate. Further enrollment was halted, and patients receiving melphalan and prednisone were offered Bortezomib for injection in addition. A later, prespecified analysis of overall survival (with median follow-up of 36.7 months with a hazard ratio of 0.65, 95% CI: 0.51, 0.84) resulted in a statistically significant survival benefit for the Bortezomib for injection, melphalan and prednisone treatment arm despite subsequent therapies including Bortezomib for injection based regimens. In an updated analysis of overall survival based on 387 deaths (median follow-up 60.1 months), the median overall survival for the Bortezomib for injection, melphalan and prednisone treatment arm was 56.4 months and for the melphalan and prednisone treatment arm was 43.1 months, with a hazard ratio of 0.695 (95% CI: 0.57, 0.85).
Table 14: Summary of Efficacy Analyses in the Previously Untreated Multiple Myeloma Study|
Efficacy Endpoint |
Bortezomib for injection**,** Melphalan and Prednisone (n=344) |
Melphalan and Prednisone (n=338) |
|
Time to Progression | ||
|
Events n (%) |
101 (29) |
152 (45) |
|
Median a (months) (95% CI) |
20.7 (17.6, 24.7) |
15.0 (14.1, 17.9) |
|
Hazard ratio b (95% CI) |
0.54 (0.42, 0.70) | |
|
p-value c |
0.000002 | |
|
Progression-Free Survival | ||
|
Events n (%) |
135 (39) |
190 (56) |
|
Median a (months) (95% CI) |
18.3 (16.6, 21.7) |
14.0 (11.1, 15.0) |
|
Hazard ratio b (95% CI) |
0.61 (0.49, 0.76) | |
|
p-value c |
0.00001 | |
|
Response Rate | ||
|
CR dn (%) |
102 (30) |
12 (4) |
|
PR dn (%) |
136 (40) |
103 (30) |
|
nCR n (%) |
5 (1) |
0 |
|
CR + PR d n (%) |
238 (69) |
115 (34) |
|
p-value e |
<10 -10 | |
|
Overall Survival at Median Follow Up of 36.7 months | ||
|
Events (deaths) n (%) |
109 (32) |
148 (44) |
|
Median a (months) (95% CI) |
Not Reached (46.2, NR) |
43.1 (34.8, NR) |
|
Hazard ratio b (95% CI) |
0.65 (0.51, 0.84) | |
|
p-value c |
0.00084 |
Note: All results are based on the analysis performed at a median follow-up
duration of 16.3 months except for the overall survival analysis.
aKaplan-Meier estimate
bHazard ratio estimate is based on a Cox proportional-hazard model adjusted for stratification factors: beta2-microglobulin, albumin, and region. A hazard ratio less than one indicates an advantage for Bortezomib for injection, melphalan and prednisone
cp-value based on the stratified log-rank test adjusted for stratification factors: beta 2-microglobulin, albumin, and region
dEBMT criteria
ep-value for Response Rate (CR + PR) from the Cochran-Mantel-Haenszel chi- square test adjusted for the stratification factors
TTP was statistically significantly longer on the Bortezomib for injection, melphalan and prednisone arm ( see Figure 1). (median follow-up 16.3 months)
|
Figure 1: Time to Progression |
|---|
|
|
Overall survival was statistically significantly longer on the Bortezomib for injection, melphalan and prednisone arm (see Figure 2). (median follow-up 60.1 months)
|
Figure 2: Overall Survival |
|---|
|
|
Randomized, Clinical Study in Relapsed Multiple Myeloma of Bortezomib for injection vs Dexamethasone
A prospective Phase 3, international, randomized (1:1), stratified, open-label clinical study (NCT00048230) enrolling 669 patients was designed to determine whether Bortezomib for injection resulted in improvement in time to progression (TTP) compared to high-dose dexamethasone in patients with progressive multiple myeloma following 1 to 3 prior therapies. Patients considered to be refractory to prior high-dose dexamethasone were excluded as were those with baseline Grade ≥2 peripheral neuropathy or platelet counts <50,000/µL. A total of 627 patients were evaluable for response.
Stratification factors were based on the number of lines of prior therapy the patient had previously received (one previous line vs more than one line of therapy), time of progression relative to prior treatment (progression during or within six months of stopping their most recent therapy vs relapse > 6 months after receiving their most recent therapy), and screening beta 2-microglobulin levels (≤2.5 mg/L vs >2.5 mg/L).
Baseline patient and disease characteristics are summarized in Table 15.
Table 15: Summary of Baseline Patient and Disease Characteristics in the Relapsed Multiple Myeloma Study|
Patient Characteristics |
Bortezomib for injection |
Dexamethasone |
|---|---|---|
|
Median age in years (range) |
62.0 (33, 84) |
61.0 (27, 86) |
|
Gender: Male/female |
56% / 44% |
60% / 40% |
|
Race: Caucasian/black/other |
90% / 6% / 4% |
88% / 7% / 5% |
|
Karnofsky performance status score ≤70 |
13% |
17% |
|
Hemoglobin <100 g/L |
32% |
28% |
|
Platelet count <75 x 10 9/L |
6% |
4% |
|
Disease Characteristics | ||
|
Type of myeloma (%): IgG/IgA/Light chain |
60% / 23% / 12% |
59% / 24% / 13% |
|
Median beta 2-microglobulin (mg/L) |
3.7 |
3.6 |
|
Median albumin (g/L) |
39.0 |
39.0 |
|
Creatinine clearance ≤30 mL/min [n (%)] |
17 (5%) |
11 (3%) |
|
Median Duration of Multiple Myeloma Since Diagnosis (Years) |
3.5 |
3.1 |
|
Number of Prior Therapeutic Lines of Treatment | ||
|
Median |
2 |
2 |
|
1 prior line |
40% |
35% |
|
60% |
65% |
|
Previous Therapy | ||
|
Any prior steroids, e.g., dexamethasone, VAD |
98% |
99% |
|
Any prior anthracyclines, e.g., VAD, mitoxantrone |
77% |
76% |
|
Any prior alkylating agents, e.g., MP, VBMCP |
91% |
92% |
|
Any prior thalidomide therapy |
48% |
50% |
|
Vinca alkaloids |
74% |
72% |
|
Prior stem cell transplant/other high-dose therapy |
67% |
68% |
|
Prior experimental or other types of therapy |
3% |
2% |
Patients in the Bortezomib for injection treatment group were to receive 8, three week treatment cycles followed by 3, five week treatment cycles of Bortezomib for injection. Patients achieving a CR were treated for four cycles beyond first evidence of CR. Within each three week treatment cycle, Bortezomib for injection 1.3 mg/m 2/dose alone was administered by intravenous bolus twice weekly for two weeks on Days 1, 4, 8, and 11 followed by a ten day rest period (Days 12 to 21). Within each five week treatment cycle, Bortezomib for injection 1.3 mg/m 2/dose alone was administered by intravenous bolus once weekly for four weeks on Days 1, 8, 15, and 22 followed by a 13 day rest period (Days 23 to 35) [ see Dosage and Administration (2.2)].
Patients in the dexamethasone treatment group were to receive 4 five week treatment cycles followed by 5, four week treatment cycles. Within each five week treatment cycle, dexamethasone 40 mg/day PO was administered once daily on Days 1 to 4, 9 to 12, and 17 to 20 followed by a 15 day rest period (Days 21 to 35). Within each four week treatment cycle, dexamethasone 40 mg/day PO was administered once daily on Days 1 to 4 followed by a 24 day rest period (Days 5 to 28). Patients with documented progressive disease on dexamethasone were offered Bortezomib for injection at a standard dose and schedule on a companion study. Following a preplanned interim analysis of time to progression, the dexamethasone arm was halted and all patients randomized to dexamethasone were offered Bortezomib for injection, regardless of disease status.
In the Bortezomib for injection arm, 34% of patients received at least one Bortezomib for injection dose in all eight of the three week cycles of therapy, and 13% received at least one dose in all 11 cycles. The average number of Bortezomib for injection doses during the study was 22, with a range of 1 to 44. In the dexamethasone arm, 40% of patients received at least one dose in all four of the five week treatment cycles of therapy, and 6% received at least one dose in all nine cycles.
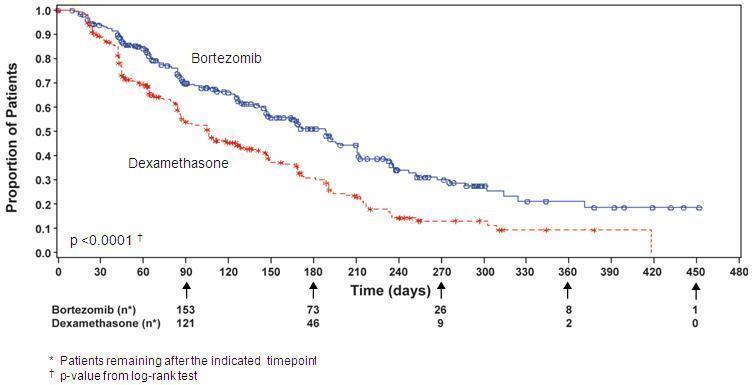
The time to event analyses and response rates from the relapsed multiple myeloma study are presented in Table 16. Response and progression were assessed using the European Group for Blood and Marrow Transplantation (EBMT) criteria. Complete response (CR) required <5% plasma cells in the marrow, 100% reduction in M-protein, and a negative immunofixation test (IF -). Partial response (PR) requires ≥50% reduction in serum myeloma protein and ≥90% reduction of urine myeloma protein on at least two occasions for a minimum of at least six weeks along with stable bone disease and normal calcium. Near complete response (nCR) was defined as meeting all the criteria for complete response including 100% reduction in M-protein by protein electrophoresis; however, M-protein was still detectable by immunofixation (IF +).
Table 16: Summary of Efficacy Analyses in the Relapsed Multiple Myeloma Study|
Efficacy Endpoint |
All Patients |
1 Prior Line of Therapy |
> 1 Prior Line of Therapy | |||
|
Bortezomib for injection |
Dex |
Bortezomib for injection |
Dex |
Bortezomib for injection |
Dex | |
|
(n=333) |
(n=336) |
(n=132) |
(n=119) |
(n=200) |
(n=217) | |
|
Time to Progression Events n (%) |
147 (44) |
196 (58) |
55 (42) |
64 (54) |
92 (46) |
132 (61) |
|
Median a (95% CI) |
6.2 mo (4.9, 6.9) |
3.5 mo (2.9, 4.2) |
7.0 mo (6.2, 8.8) |
5.6 mo (3.4, 6.3) |
4.9 mo (4.2, 6.3) |
2.9 mo 2.8, 3.5) |
|
Hazard ratio b à (95% CI) |
0.55 (0.44, 0.69) |
0.55 (0.38, 0.81) |
0.54 (0.41, 0.72) | |||
|
p-value c |
<0.0001 |
0.0019 |
<0.0001 | |||
|
Overall Survival Events (deaths) n (%) |
51 (15) |
84 (25) |
12 (9) |
24 (20) |
39 (20) |
60 (28) |
|
Hazard ratio b (95% CI) |
0.57 (0.40, 0.81) |
0.39 (0.19, 0.81) |
0.65 (0.43, 0.97) | |||
|
p-value c,d |
<0.05 |
<0.05 |
<0.05 | |||
|
Response Rate Population e n = 627 |
n=315 |
n=312 |
n=128 |
n=110 |
n=187 |
n=202 |
|
CR fn (%) |
20 (6) |
2 (<1) |
8 (6) |
2 (2) |
12 (6) |
0 (0) |
|
PR f n (%) |
101 (32) |
54 (17) |
49 (38) |
27 (25) |
52 (28) |
27 (13) |
|
nCR f,g n (%) |
21 (7) |
3 (<1) |
8 (6) |
2 (2) |
13 (7) |
1 (<1) |
|
CR + PR f n (%) |
121 (38) |
56 (18) |
57 (45) |
29 (26) |
64 (34) |
27 (13) |
|
p-value h |
<0.0001 |
0.0035 |
<0.0001 |
a Kaplan-Meier estimate
b Hazard ratio is based on Cox proportional-hazard model with the treatment as
single independent variable. A hazard ratio less than one indicates an
advantage for Bortezomib for injection
c p-value based on the stratified log-rank test including randomization
stratification factors
d Precise p-value cannot be rendered
e Response population includes patients who had measurable disease at baseline
and received at least one dose of study drug
f EBMT criteria; nCR meets all EBMT criteria for CR but has positive IF. Under
EBMT criteria nCR is in the PR category
g In two patients, the IF was unknown
h p-value for Response Rate (CR + PR) from the Cochran-Mantel-Haenszel chi-
square test adjusted for the stratification factors
TTP was statistically significantly longer on the Bortezomib for injection arm (see Figure 3).
|
Figure 3: Time to Progression |
|---|
|
|
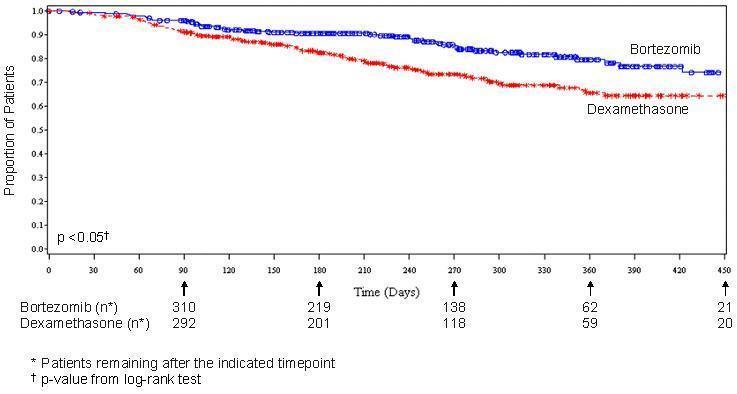
As shown in Figure 4 Bortezomib for injection had a significant survival advantage relative to dexamethasone (p < 0.05). The median follow-up was 8.3 months.
|
Figure 4: Overall Survival |
|---|
|
|
For the 121 patients achieving a response (CR or PR) on the Bortezomib for injection arm, the median duration was 8.0 months (95% CI: 6.9, 11.5 months) compared to 5.6 months (95% CI: 4.8, 9.2 months) for the 56 responders on the dexamethasone arm. The response rate was significantly higher on the Bortezomib for injection arm regardless of beta2-microglobulin levels at baseline.
Randomized, Open-Label Clinical Study of Bortezomib for injection Subcutaneous vs Intravenous in Relapsed Multiple Myeloma
An open-label, randomized, Phase 3 noninferiority study (NCT00722566) compared the efficacy and safety of the subcutaneous administration of Bortezomib for injection vs the intravenous administration. This study included 222 bortezomib naïve patients with relapsed multiple myeloma, who were randomized in a 2:1 ratio to receive 1.3 mg/m 2 of Bortezomib for injection by either the subcutaneous (n=148) or intravenous (n=74) route for eight cycles. Patients who did not obtain an optimal response (less than Complete Response (CR)) to therapy with Bortezomib for injection alone after four cycles were allowed to receive oral dexamethasone 20 mg daily on the day of and after Bortezomib for injection administration (82 patients in subcutaneous treatment group and 39 patients in the intravenous treatment group). Patients with baseline Grade ≥2 peripheral neuropathy or neuropathic pain, or platelet counts <50,000/µL were excluded. A total of 218 patients were evaluable for response.
Stratification factors were based on the number of lines of prior therapy the patient had received (one previous line vs more than one line of therapy), and international staging system (ISS) stage (incorporating beta 2-microglobulin and albumin levels; Stages I, II, or III).
The baseline demographic and other characteristics of the two treatment groups are summarized as follows: the median age of the patient population was approximately 64 years of age (range 38 to 88 years), primarily male (subcutaneous: 50%, intravenous: 64%); the primary type of myeloma is IgG (subcutaneous: 65% IgG, 26% IgA, 8% light chain; intravenous: 72% IgG, 19% IgA, 8% light chain), ISS staging I/II/III (%) was 27, 41, 32 for both subcutaneous and intravenous, Karnofsky performance status score was ≤ 70% in 22% of subcutaneous and 16% of intravenous, creatinine clearance was 67.5 mL/min in subcutaneous and 73 mL/min in intravenous, the median years from diagnosis was 2.68 and 2.93 in subcutaneous and intravenous respectively and the proportion of patients with more than one prior line of therapy was 38% in subcutaneous and 35% in intravenous.
This study met its primary (noninferiority) objective that single agent subcutaneous Bortezomib for injection retains at least 60% of the overall response rate after four cycles relative to single agent intravenous Bortezomib for injection. The results are provided in Table 17.
Table 17: Summary of Efficacy Analyses in the Relapsed Multiple Myeloma Study of Bortezomib for injection Subcutaneous versus Intravenous|
Subcutaneous Bortezomib for injection |
Intravenous Bortezomib for injection | |
|---|---|---|
|
Intent to Treat Population |
(n=148) |
(n=74) |
|
Primary Endpoint | ||
|
** Response Rate at 4 Cycles** | ||
|
ORR (CR + PR) n(%) |
63 (43) |
31 (42) |
|
Ratio of Response Rates (95% CI) |
1.01 (0.73, 1.40) | |
|
CR n (%) |
11 (7) |
6 (8) |
|
PR n (%) |
52 (35) |
25 (34) |
|
nCR n (%) |
9 (6) |
4 (5) |
|
Secondary Endpoints | ||
|
Response Rate at 8 Cycles | ||
|
ORR (CR + PR) |
78 (53) |
38 (51) |
|
CR n (%) |
17 (11) |
9 (12) |
|
PR n (%) |
61 (41) |
29 (39) |
|
nCR n (%) |
14 (9) |
7 (9) |
|
Median Time to Progression, months |
10.4 |
9.4 |
|
Median Progression-Free Survival, months |
10.2 |
8.0 |
|
**1 Year Overall Survival (%)**a |
72.6 |
76.7 |
aMedian duration of follow up is 11.8 months
A Randomized Phase 2 Dose-Response Study in Relapsed Multiple Myeloma
An open-label, multicenter study randomized 54 patients with multiple myeloma who had progressed or relapsed on or after front-line therapy to receive Bortezomib for injection 1 mg/m 2 or 1.3 mg/m 2 intravenous bolus twice weekly for two weeks on Days 1, 4, 8, and 11 followed by a ten day rest period (Days 12 to 21). The median duration of time between diagnosis of multiple myeloma and first dose of Bortezomib for injection on this trial was two years, and patients had received a median of one prior line of treatment (median of three prior therapies). A single complete response was seen at each dose. The overall response rates (CR + PR) were 30% (8/27) at 1 mg/m 2 and 38% (10/26) at 1.3 mg/m 2.
A Phase 2 Open-Label Extension Study in Relapsed Multiple Myeloma
Patients from the two Phase 2 studies, who in the investigators' opinion would experience additional clinical benefit, continued to receive Bortezomib for injection beyond 8 cycles on an extension study. Sixty-three (63) patients from the Phase 2 multiple myeloma studies were enrolled and received a median of seven additional cycles of Bortezomib for injection therapy for a total median of 14 cycles (range 7 to 32). The overall median dosing intensity was the same in both the parent protocol and extension study. Sixty-seven percent (67%) of patients initiated the extension study at the same or higher dose intensity at which they completed the parent protocol, and 89% of patients maintained the standard three week dosing schedule during the extension study. No new cumulative or new long-term toxicities were observed with prolonged Bortezomib for injection treatment [ see Adverse Reactions (6.1)].
A Single-Arm Trial of Retreatment in Relapsed Multiple Myeloma
A single arm, open-label trial (NCT00431769) was conducted to determine the
efficacy and safety of retreatment with Bortezomib for injection. One hundred
and thirty patients (≥18 years of age) with multiple myeloma who previously
had at least partial response on a Bortezomib for injection-containing regimen
(median of two prior lines of therapy [range 1 to 7]) were retreated upon
progression with Bortezomib for injection administered intravenously. Patients
were excluded from trial participation if they had peripheral neuropathy or
neuropathic pain of Grade ≥2. At least six months after prior Bortezomib for
injection therapy, Bortezomib for injection was restarted at the last
tolerated dose of 1.3 mg/m2 (n=93) or ≤1 mg/m2 (n=37) and given on Days 1, 4,
8 and 11 every three weeks for maximum of eight cycles either as single agent
or in combination with dexamethasone in accordance with the standard of care.
Dexamethasone was administered in combination with Bortezomib for injection to
83 patients in Cycle 1 with an additional 11 patients receiving dexamethasone
during the course of Bortezomib for injection retreatment cycles.
The primary endpoint was best confirmed response to retreatment as assessed by European Group for Blood and Marrow Transplantation (EBMT) criteria. Fifty of the 130 patients achieved a best confirmed response of Partial Response or better for an overall response rate of 38.5% (95% CI: 30.1, 47.4). One patient achieved a Complete Response and 49 achieved Partial Response. In the 50 responding patients, the median duration of response was 6.5 months and the range was 0.6 to 19.3 months.
14.2 Mantle Cell Lymphoma
A Randomized, Open-Label Clinical Study in Patients with Previously Untreated
Mantle Cell Lymphoma
A randomized, open-label, Phase 3 study (NCT00722137) was conducted in 487
adult patients with previously untreated mantle cell lymphoma (Stage II, III
or IV) who were ineligible or not considered for bone marrow transplantation
to determine whether Bortezomib for injection administered in combination with
rituximab, cyclophosphamide, doxorubicin, and prednisone (VcR-CAP) resulted in
improvement in progression free survival (PFS) when compared to the
combination of rituximab, cyclophosphamide, doxorubicin, vincristine, and
prednisone (R-CHOP). This clinical study utilized independent pathology
confirmation and independent radiologic response assessment.
Patients in the VcR-CAP treatment arm received Bortezomib for injection (1.3 mg/m 2) administered intravenously on Days 1, 4, 8, and 11 (rest period Days 12 to 21); rituximab (375 mg/m 2) on Day 1; cyclophosphamide (750 mg/m 2) on Day 1; doxorubicin (50 mg/m 2) on Day 1; and prednisone (100 mg/m 2) on Day 1 through Day 5 of the 21 day treatment cycle. For patients with a response first documented at Cycle 6, two additional treatment cycles were allowed.
Median patient age was 66 years, 74% were male, 66% were Caucasian and 32% were Asian. Sixty nine percent of patients had a positive bone marrow aspirate and/or a positive bone marrow biopsy for MCL, 54% of patients had an International Prognostic Index (IPI) score of three (high intermediate) or higher and 76% had Stage IV disease.
The majority of the patients in both groups received six or more cycles of treatment, 84% in the VcR-CAP group and 83% in the R-CHOP group. Median number of cycles received by patients in both treatment arms was six with 17% of patients in the R-CHOP group and 14% of subjects in the VcR-CAP group receiving up to two additional cycles.
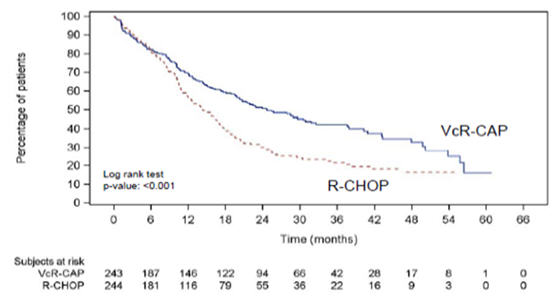
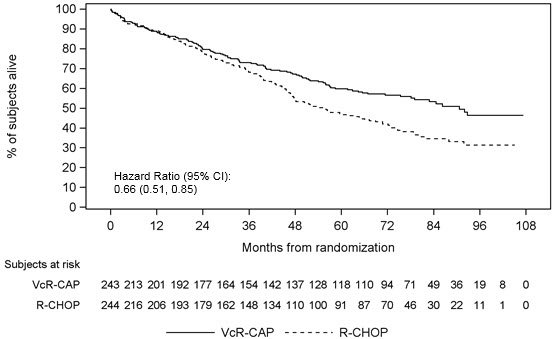
The efficacy results for PFS, CR and ORR with a median follow-up of 40 months are presented in Table 18. The response criteria used to assess efficacy were based on the International Workshop to Standardize Response Criteria for Non- Hodgkin’s Lymphoma (IWRC). Final overall survival results at a median follow- up of 78.5 months are also presented in Table 18 and Figure 6. The combination of VcR-CAP resulted in statistically significant prolongation of PFS compared with R-CHOP (see Table 18, Figure 5).
Table 18: Summary of Efficacy Analyses in the Previously Untreated Mantle Cell Lymphoma Study|
Efficacy Endpoint |
VcR-CAP |
R-CHOP |
|---|---|---|
|
Note: All results are based on the analysis performed at a median follow-up duration of 40 months except for the overall survival analysis, which was performed at a median follow-up of 78.5 months. CI = Confidence Interval; IPI = International Prognostic Index; LDH = Lactate
dehydrogenase | ||
|
Progression-Free Survival (by independent radiographic assessment) | ||
|
Events n (%) |
133 (55) |
165 (68) |
|
Median a (months) |
25 |
14 |
|
Hazard ratio b |
0.63 | |
|
p-value c |
<0.001 | |
|
Complete Response Rate(CR) d | ||
|
n (%) |
108 (44) |
82 (34) |
|
**Overall Response Rate (CR + Cru + PR)**e | ||
|
n (%) |
214 (88) |
208 (85) |
|
(95% CI) |
(83, 92) |
(80, 89) |
|
Overall Survival | ||
|
Events n(%) |
103 (42) |
138 (57) |
|
Median a (months) |
91 |
56 |
|
Hazard Ratio b |
0.66 |
Key: R-CHOP = rituximab, cyclophosphamide, doxorubicin, vincristine, and
prednisone;
VcR-CAP = VELCADE, rituximab, cyclophosphamide, doxorubicin, and prednisone.
Key: R-CHOP = rituximab, cyclophosphamide, doxorubicin, vincristine, and
prednisone;
VcR-CAP = VELCADE, rituximab, cyclophosphamide, doxorubicin, and prednisone
A Phase 2 Single-Arm Clinical Study in Relapsed Mantle Cell Lymphoma after
Prior Therapy****
The safety and efficacy of Bortezomib for injection in relapsed or refractory
mantle cell lymphoma were evaluated in an open-label, single-arm, multicenter
study (NCT00063713) of 155 patients with progressive disease who had received
at least one prior therapy. The median age of the patients was 65 years (42,
89), 81% were male, and 92% were Caucasian. Of the total, 75% had one or more
extra-nodal sites of disease, and 77% were Stage 4. In 91% of the patients,
prior therapy included all of the following: an anthracycline or mitoxantrone,
cyclophosphamide, and rituximab. A total of thirty seven percent (37%) of
patients were refractory to their last prior therapy. An intravenous bolus
injection of Bortezomib for injection 1.3 mg/m2/dose was administered twice
weekly for two weeks on Days 1, 4, 8, and 11 followed by a ten day rest period
(Days 12 to 21) for a maximum of 17 treatment cycles. Patients achieving a CR
or CRu were treated for four cycles beyond first evidence of CR or CRu. The
study employed dose modifications for toxicity[ see Dosage and Administration (2.6, 2.7) ]
Responses to Bortezomib for injection are shown in Table 19. Response rates to Bortezomib for injection were determined according to the International Workshop Response Criteria (IWRC) based on independent radiologic review of CT scans. The median number of cycles administered across all patients was four; in responding patients the median number of cycles was eight. The median time to response was 40 days (range: 31 to 204 days). The median duration of follow-up was more than 13 months.
Table 19: Response Outcomes in a Phase 2 Relapsed Mantle Cell Lymphoma Study|
Response Analyses (N = 155) |
N (%) |
95% CI |
|---|---|---|
|
Overall Response Rate (IWRC) (CR + CRu + PR) |
48 (31) |
(24, 39) |
|
Complete Response (CR + CRu) |
12 (8) |
(4, 13) |
|
CR |
10 (6) |
(3, 12) |
|
CRu |
2 (1) |
(0, 5) |
|
Partial Response (PR) |
36 (23) |
(17, 31) |
|
Duration of Response |
Median |
95% CI |
|
CR + CRu + PR (N = 48) |
9.3 months |
(5.4, 13.8) |
|
CR + CRu (N = 12) |
15.4 months |
(13.4, 15.4) |
|
PR (N=36) |
6.1 months |
(4.2, 9.3) |
REFERENCES SECTION
15 REFERENCES
- "OSHA Hazardous Drugs" (refer to antineoplastic weblinks including OSHA Technical Manual). OSHA. http://www.osha.gov/SLTC/hazardousdrugs/index.html
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
Bortezomib for injection , 3.5 mg/vialis supplied as individually cartoned 10
mL vials containing 3.5 mg of bortezomib as a white to off-white cake or
powder.
NDC 51817-586-01
3.5 mg single-dose vial
Unopened vials may be stored at controlled room temperature 25°C (77°F); excursions permitted from 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature]. Retain in original package to protect from light.
Follow guidelines for handling and disposal for hazardous drugs, including the use of gloves and other protective clothing to prevent skin contact 1.
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Discuss the following with patients prior to treatment with Bortezomib for injection :
Peripheral Neuropathy Advise patients to report the development or worsening of sensory and motor peripheral neuropathy to their healthcare provider [ see Warnings and Precautions (5.1)].
Hypotension Advise patients to drink adequate fluids to avoid dehydration and
to report symptoms of hypotension to their healthcare provider [ see Warnings and Precautions (5.2)].
Instruct patients to seek medical advice if they experience symptoms of
dizziness, light headedness or fainting spells, or muscle cramps.
Cardiac Toxicity Advise patients to report signs or symptoms of heart failure to their healthcare provider [ see Warnings and Precautions (5.3)].
Pulmonary Toxicity Advise patients to report symptoms of ARDS, pulmonary hypertension, pneumonitis, and pneumonia immediately to their healthcare provider [ see Warnings and Precautions (5.4)].
Posterior Reversible Encephalopathy Syndrome (PRES) Advise patients to seek immediate medical attention for signs or symptoms of PRES [ see Warnings and Precautions (5.5)].
Gastrointestinal Toxicity Advise patients to report symptoms of gastrointestinal toxicity to their healthcare provider and to drink adequate fluids to avoid dehydration. Instruct patients to seek medical advice if they experience symptoms of dizziness, light headedness or fainting spells, or muscle cramps [ see Warnings and Precautions (5.6)].
T hrombocytopenia/Neutropenia Advise patients to report signs or symptoms of bleeding or infection immediately to their healthcare provider [ see Warnings and Precautions (5.7)].
Tumor Lysis Syndrome Advise patients of the risk of tumor lysis syndrome and to drink adequate fluids to avoid dehydration [ see Warnings and Precautions (5.8)].
Hepatic Toxicity Advise patients to report signs or symptoms of hepatic toxicity to their healthcare provider [ see Warnings and Precautions (5.9)].
Thrombotic Microangiopathy
Advise patients to seek immediate medical attention if any signs or symptoms
of thrombotic microangiopathy occur [ see Warnings and Precautions (5.10)].
Ability to Drive or Operate Machinery or Impairment of Mental Ability
Bortezomib for injection may cause fatigue, dizziness, syncope,
orthostatic/postural hypotension. Advise patients not to drive or operate
machinery if they experience any of these symptoms [ see Warnings and Precautions (5.2, 5.5)].
Embryo-Fetal Toxicity
Advise females of the potential risk to the fetus and to use effective
contraception during treatment with Bortezomib for injection and for seven
months following the last dose. Advise male patients with female partners of
reproductive potential to use effective contraception during treatment with
Bortezomib for injection and for four months following the last dose. Instruct
patients to report pregnancy to their physicians immediately if they or their
female partner becomes pregnant during treatment or within seven months
following last dose [ see Warnings and Precautions (5.11)].
Lactation
Advise women not to breastfeed while receiving Bortezomib for injection and
for two months after last dose [ see Use in Specific Populations (8.2)].
Concomitant Medications
Advise patients to speak with their physicians about any other medication they
are currently taking.
Diabetic Patients
Advise patients to check their blood sugar frequently if using an oral
antidiabetic medication and to notify their physicians of any changes in blood
sugar level.
Dermal
Advise patients to contact their physicians if they experience rash, severe
injection site reactions [ see Dosage and Administration (2.9)], or skin pain.
Discuss with patients the option for antiviral prophylaxis for herpes virus
infection [ see Adverse Reactions (6.1) ].
Other
Instruct patients to contact their physicians if they develop an increase in
blood pressure, bleeding, fever, constipation, or decreased appetite.
SPL UNCLASSIFIED SECTION
Manufactured by: Pharmascience Inc.
100 Boul. de l’Industrie, Candiac, QC, Canada, J5R 1J1
Company Logo
Revised: November 2021 (Rev 07)
PRINCIPAL DISPLAY PANEL - 3.5 mg Carton Label
Reconstitution Information
SUBCUTANEOUS INJECTION ONLY
0.9% NaCl
Add 1.4 mL
0.9% Sodium Chloride
To make 2.5 mg / mL final concentration