Nystatin
Nystatin Cream USP, 100,000 units per gram FOR TOPICAL USE ONLY. NOT FOR OPHTHALMIC USE
f4c298d5-b012-7b56-e053-2a95a90a3d55
HUMAN PRESCRIPTION DRUG LABEL
Aug 28, 2025
RedPharm Drug, Inc
DUNS: 828374897
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Nystatin
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (12)
Drug Labeling Information
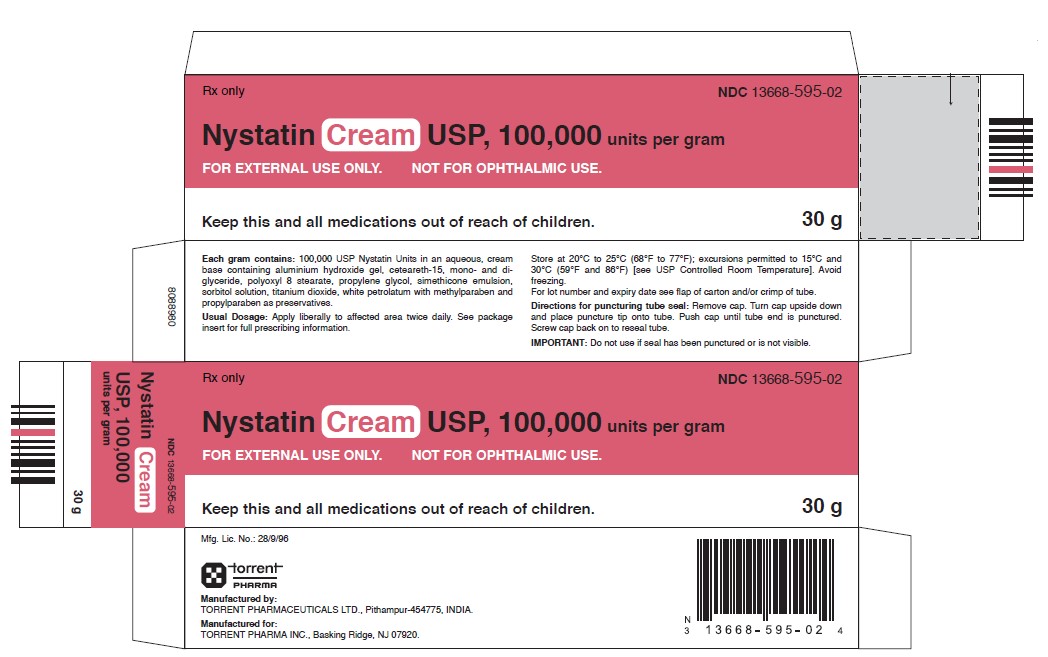
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL



DESCRIPTION SECTION
DESCRIPTION
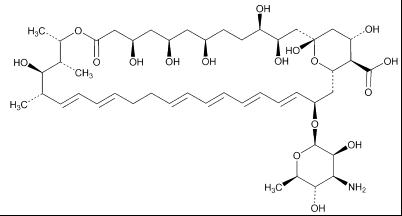
Nystatin is a polyene antifungal antibiotic obtained from Streptomyces nursei.
Structural formula:
|
** Molecular formula** |
** :** |
C 47H 75NO 17 |
|
** Molecular weight** |
** :** |
926.09 g/mol |
Nystatin cream is for dermatologic use.
Nystatin cream, USP for topical use, contains 100,000 USP nystatin units per gram. Inactive ingredients: aluminium hydroxide gel, ceteareth-15, mono- and di- glyceride, polyoxyl 8 stearate, propylene glycol, simethicone emulsion, sorbitol solution, titanium dioxide, white petrolatum, methylparaben and propylparaben.
STORAGE AND HANDLING SECTION
STORAGE AND HANDLING
Store at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C and 30°C (59°F and 86°F) [see USP Controlled Room Temperature]. Avoid freezing.

Manufactured by:
TORRENT PHARMACEUTICALS LTD., Pithampur-454775, INDIA**.**
Manufactured for:
TORRENT PHARMA INC., Basking Ridge, NJ 07920
8088981 August 2022
