Visine Red Eye Hydrating Comfort
VISINE Red Eye Hydrating Comfort
dcd4164c-aa1e-4f07-ab2c-b439275629db
HUMAN OTC DRUG LABEL
Jun 16, 2025
Morning Star OTC
DUNS: 078589357
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
TETRAHYDROZOLINE HYDROCHLORIDE and Polyethylene Glycol 400
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (15)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
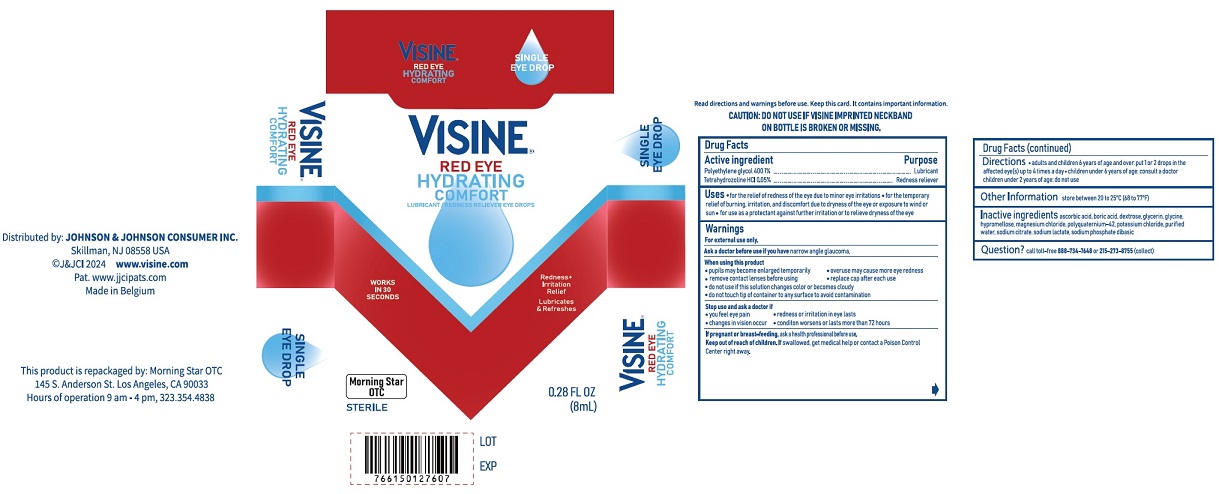
INDICATIONS & USAGE SECTION
Uses
- for the relief of redness of the eye due to minor eye irritations
- for the temporary relief of burning, irritation, and discomfort due to dryness of the eye or exposure to wind or sun
- for use as a protectant against further irritation or to relieve dryness of the eye
INACTIVE INGREDIENT SECTION
Inactive ingredients
ascorbic acid, boric acid, dextrose, glycerin, glycine, hypromellose, magnesium chloride, polyquaternium-42, potassium chloride, purified water, sodium citrate, sodium lactate, sodium phosphate dibasic
OTC - QUESTIONS SECTION
Questions?
call toll-free888-734-7648or215-273-8755(collect)
SPL UNCLASSIFIED SECTION
Repackaged by:
Morning Star OTC
145 S. Anderson St, Los Angeles,
CA 90033
OTC - ACTIVE INGREDIENT SECTION
|
Active ingredients |
Purpose |
|---|---|
|
Polyethylene glycol 400 1% |
Lubricant |
|
Tetrahydrozoline HCl 0.05% |
Redness reliever |
WARNINGS SECTION
Warnings
For external use only.
Ask a doctor before use if you havenarrow angle glaucoma.
When using this product
- pupils may become enlarged temporarily
- overuse may cause more eye redness
- remove contact lenses before using
- do not use if this solution changes color or becomes cloudy
- do not touch tip of container to any surface to avoid contamination
- replace cap after each use
Stop use and ask a doctor if
- you feel eye pain
- changes in vision occur
- redness or irritation of the eye lasts
- condition worsens or lasts more than 72 hours
**If pregnant or breast-feeding,**ask a health professional before use.
**Keep out of reach of children.**If swallowed, get medical help or contact a Poison Control Center right away.
DOSAGE & ADMINISTRATION SECTION
Directions
- adults and children 6 years of age and over: put 1 or 2 drops in the affected eye(s) up to 4 times a day
- children under 6 years of age: consult a doctor
- children under 2 years of age: do not use
STORAGE AND HANDLING SECTION
Other information
Store at 20° to 25°C (68° to 77°F)
