Fluticasone Propionate and Salmeterol HFA
These highlights do not include all the information needed to use Fluticasone Propionate and Salmeterol HFA safely and effectively. See full prescribing information for Fluticasone Propionate and Salmeterol HFA. Fluticasone Propionate and Salmeterol HFA inhalation aerosol, for oral inhalation useInitial U.S. Approval: 2000
666c5b12-a4f7-4c53-8261-4742b5e5a6df
HUMAN PRESCRIPTION DRUG LABEL
Aug 1, 2022
Prasco Laboratories
DUNS: 065969375
Products 3
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
fluticasone propionate and salmeterol xinafoate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (3)
fluticasone propionate and salmeterol xinafoate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (3)
fluticasone propionate and salmeterol xinafoate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (3)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
NDC 66993-088-96
Fluticasone Propionate and SalmeterolHFA Inhalation Aerosol
230 mcg/21 mcg
PRASCO
For oral inhalation with Fluticasone Propionate and Salmeterol HFA actuator only.
**Contents:**Each canister contains a microcrystalline suspension of fluticasone propionate and salmeterol xinafoate in propellant HFA-134a (1,1,1,2-tetrafluoroethane).
Each actuation delivers 230 mcg of fluticasone propionate and 30.45 mcg of salmeterol xinafoate equivalent to 21 mcg of salmeterol base from the mouthpiece.
See prescribing information for dosage information.
RX Only
120 Metered Actuations
Net Wt. 12 g
6200000083189 Rev. 10/22

WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Serious Asthma-Related Events – Hospitalizations, Intubations, Death
Use of LABA as monotherapy (without ICS) for asthma is associated with an increased risk of asthma-related death [see Salmeterol Multicenter Asthma Research Trial (SMART)]. Available data from controlled clinical trials also suggest that use of LABA as monotherapy increases the risk of asthma-related hospitalization in pediatric and adolescent patients. These findings are considered a class effect of LABA monotherapy. When LABA are used in fixed- dose combination with ICS, data from large clinical trials do not show a significant increase in the risk of serious asthma-related events (hospitalizations, intubations, death) compared with ICS alone (see Serious Asthma-Related Events with Inhaled Corticosteroid/Long-acting Beta2-adrenergic Agonists).
Serious Asthma-Related Events with Inhaled Corticosteroid/Long-acting Beta2-adrenergic Agonists
Four (4) large, 26-week, randomized, double-blind, active-controlled clinical safety trials were conducted to evaluate the risk of serious asthma-related events when LABA were used in fixed‑dose combination with ICS compared with ICS alone in subjects with asthma. Three (3) trials included adult and adolescent subjects aged 12 years and older: 1 trial compared fluticasone propionate/salmeterol inhalation powder with fluticasone propionate inhalation powder, 1 trial compared mometasone furoate/formoterol with mometasone furoate, and 1 trial compared budesonide/formoterol with budesonide. The fourth trial included pediatric subjects aged 4 to 11 years and compared fluticasone propionate/salmeterol inhalation powder with fluticasone propionate inhalation powder. The primary safety endpoint for all 4 trials was serious asthma-related events (hospitalizations, intubations, death). A blinded adjudication committee determined whether events were asthma related.
The 3 adult and adolescent trials were designed to rule out a risk margin of 2.0, and the pediatric trial was designed to rule out a risk margin of 2.7. Each individual trial met its pre-specified objective and demonstrated non- inferiority of ICS/LABA to ICS alone. A meta-analysis of the 3 adult and adolescent trials did not show a significant increase in risk of a serious asthma-related event with ICS/LABA fixed-dose combination compared with ICS alone (Table 1). These trials were not designed to rule out all risk for serious asthma-related events with ICS/LABA compared with ICS.
Table 1. Meta-analysis of Serious Asthma-Related Events in Subjects with Asthma Aged 12 Years and Older|
ICS = Inhaled Corticosteroid, LABA = Long-acting Beta2-adrenergic Agonist. | |||
|
ICS/LABA **(n = 17,537)**a |
ICS **(n = 17,552)**a |
ICS/LABA vs. ICS Hazard Ratio **(95% CI)**b | |
|
Serious asthma-related eventc |
116 |
105 |
1.10 (0.85, 1.44) |
|
Asthma-related death |
2 |
0 | |
|
Asthma-related intubation (endotracheal) |
1 |
2 | |
|
Asthma-related hospitalization (≥24-hour stay) |
115 |
105 |
The pediatric safety trial included 6,208 pediatric subjects aged 4 to 11 years who received ICS/LABA (fluticasone propionate/salmeterol inhalation powder) or ICS (fluticasone propionate inhalation powder). In this trial, 27/3,107 (0.9%) subjects randomized to ICS/LABA and 21/3,101 (0.7%) subjects randomized to ICS experienced a serious asthma-related event. There were no asthma-related deaths or intubations. ICS/LABA did not show a significantly increased risk of a serious asthma-related event compared with ICS based on the pre-specified risk margin (2.7), with an estimated hazard ratio of time to first event of 1.29 (95% CI: 0.73, 2.27).
Salmeterol Multicenter Asthma Research Trial (SMART)
A 28-week, placebo-controlled, U.S. trial that compared the safety of salmeterol with placebo, each added to usual asthma therapy, showed an increase in asthma-related deaths in subjects receiving salmeterol (13/13,176 in subjects treated with salmeterol versus 3/13,179 in subjects treated with placebo; relative risk: 4.37 [95% CI: 1.25, 15.34]). Use of background ICS was not required in SMART. The increased risk of asthma‑related death is considered a class effect of LABA monotherapy.
5.2 Deterioration of Disease and Acute Episodes
Fluticasone Propionate and Salmeterol HFA should not be initiated in patients during rapidly deteriorating or potentially life-threatening episodes of asthma. Fluticasone propionate and salmeterol HFA has not been studied in subjects with acutely deteriorating asthma. The initiation of Fluticasone Propionate and Salmeterol HFA in this setting is not appropriate.
Serious acute respiratory events, including fatalities, have been reported when salmeterol, a component of Fluticasone Propionate and Salmeterol HFA, has been initiated in patients with significantly worsening or acutely deteriorating asthma. In most cases, these have occurred in patients with severe asthma (e.g., patients with a history of corticosteroid dependence, low pulmonary function, intubation, mechanical ventilation, frequent hospitalizations, previous life-threatening acute asthma exacerbations) and in some patients with acutely deteriorating asthma (e.g., patients with significantly increasing symptoms; increasing need for inhaled, short-acting beta2-agonists; decreasing response to usual medications; increasing need for systemic corticosteroids; recent emergency room visits; deteriorating lung function). However, these events have occurred in a few patients with less severe asthma as well. It was not possible from these reports to determine whether salmeterol contributed to these events.
Increasing use of inhaled, short-acting beta2-agonists is a marker of deteriorating asthma. In this situation, the patient requires immediate reevaluation with reassessment of the treatment regimen, giving special consideration to the possible need for replacing the current strength of Fluticasone Propionate and Salmeterol HFA with a higher strength, adding additional ICS, or initiating systemic corticosteroids. Patients should not use more than 2 inhalations twice daily of Fluticasone Propionate and Salmeterol HFA.
Fluticasone Propionate and Salmeterol HFA should not be used for the relief of acute symptoms, i.e., as rescue therapy for the treatment of acute episodes of bronchospasm. Fluticasone propionate and salmeterol HFA has not been studied in the relief of acute symptoms and extra doses should not be used for that purpose. Acute symptoms should be treated with an inhaled, short-acting beta2-agonist.
When beginning treatment with Fluticasone Propionate and Salmeterol HFA, patients who have been taking oral or inhaled, short-acting beta2-agonists on a regular basis (e.g., 4 times a day) should be instructed to discontinue the regular use of these drugs.
5.3 Avoid Excessive Use of Fluticasone Propionate and Salmeterol HFA and
Avoid Use with Other Long-acting Beta2-agonists
Fluticasone Propionate and Salmeterol HFA should not be used more often than recommended, at higher doses than recommended, or in conjunction with other medicines containing LABA, as an overdose may result. Clinically significant cardiovascular effects and fatalities have been reported in association with excessive use of inhaled sympathomimetic drugs. Patients using Fluticasone Propionate and Salmeterol HFA should not use another medicine containing a LABA (e.g., salmeterol, formoterol fumarate, arformoterol tartrate, indacaterol) for any reason.
5.4 Oropharyngeal Candidiasis
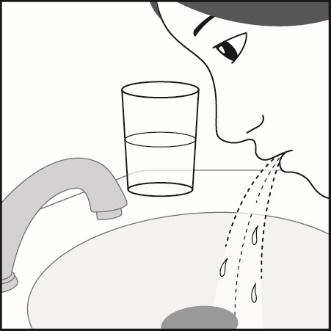
In clinical trials, the development of localized infections of the mouth and pharynx with Candida albicans has occurred in subjects treated with fluticasone propionate and salmeterol HFA. When such an infection develops, it should be treated with appropriate local or systemic (i.e., oral) antifungal therapy while treatment with Fluticasone Propionate and Salmeterol HFA continues, but at times therapy with Fluticasone Propionate and Salmeterol HFA may need to be interrupted. Advise the patient to rinse his/her mouth with water without swallowing following inhalation to help reduce the risk of oropharyngeal candidiasis.
5.5 Pneumonia
Lower respiratory tract infections, including pneumonia, have been reported in patients with chronic obstructive pulmonary disease (COPD) following the inhaled administration of corticosteroids, including fluticasone propionate and ADVAIR DISKUS (fluticasone propionate and salmeterol inhalation powder). In 2 replicate 1-year trials in 1,579 subjects with COPD, there was a higher incidence of pneumonia reported in subjects receiving ADVAIR DISKUS 250 mcg/50 mcg (7%) than in those receiving salmeterol 50 mcg (3%). The incidence of pneumonia in the subjects treated with ADVAIR DISKUS was higher in subjects older than 65 years (9%) compared with the incidence in subjects younger than 65 years (4%).
In a 3-year trial in 6,184 subjects with COPD, there was a higher incidence of pneumonia reported in subjects receiving ADVAIR DISKUS 500 mcg/50 mcg compared with placebo (16% with ADVAIR DISKUS 500 mcg/50 mcg, 14% with fluticasone propionate 500 mcg, 11% with salmeterol 50 mcg, and 9% with placebo). Similar to what was seen in the 1-year trials with ADVAIR DISKUS 250 mcg/50 mcg, the incidence of pneumonia was higher in subjects older than 65 years (18% with ADVAIR DISKUS 500 mcg/50 mcg versus 10% with placebo) compared with subjects younger than 65 years (14% with ADVAIR DISKUS 500 mcg/50 mcg versus 8% with placebo).
5.6 Immunosuppression and Risk of Infections
Persons who are using drugs that suppress the immune system are more susceptible to infections than healthy individuals. Chickenpox and measles, for example, can have a more serious or even fatal course in susceptible children or adults using corticosteroids. In such children or adults who have not had these diseases or been properly immunized, particular care should be taken to avoid exposure. How the dose, route, and duration of corticosteroid administration affect the risk of developing a disseminated infection is not known. The contribution of the underlying disease and/or prior corticosteroid treatment to the risk is also not known. If a patient is exposed to chickenpox, prophylaxis with varicella zoster immune globulin (VZIG) may be indicated. If a patient is exposed to measles, prophylaxis with pooled intramuscular immunoglobulin (IG) may be indicated. (See the respective package inserts for complete VZIG and IG prescribing information.) If chickenpox develops, treatment with antiviral agents may be considered.
ICS should be used with caution, if at all, in patients with active or quiescent tuberculosis infections of the respiratory tract; systemic fungal, bacterial, viral, or parasitic infections; or ocular herpes simplex.
5.7 Transferring Patients from Systemic Corticosteroid Therapy
HPA Suppression/Adrenal Insufficiency
Particular care is needed for patients who have been transferred from systemically active corticosteroids to ICS because deaths due to adrenal insufficiency have occurred in patients with asthma during and after transfer from systemic corticosteroids to less systemically available ICS. After withdrawal from systemic corticosteroids, a number of months are required for recovery of hypothalamic-pituitary-adrenal (HPA) function.
Patients who have been previously maintained on 20 mg or more of prednisone (or its equivalent) may be most susceptible, particularly when their systemic corticosteroids have been almost completely withdrawn. During this period of HPA suppression, patients may exhibit signs and symptoms of adrenal insufficiency when exposed to trauma, surgery, or infection (particularly gastroenteritis) or other conditions associated with severe electrolyte loss. Although Fluticasone Propionate and Salmeterol HFA may control asthma symptoms during these episodes, in recommended doses it supplies less than normal physiological amounts of glucocorticoid systemically and does NOT provide the mineralocorticoid activity that is necessary for coping with these emergencies.
During periods of stress or a severe asthma attack, patients who have been withdrawn from systemic corticosteroids should be instructed to resume oral corticosteroids (in large doses) immediately and to contact their physicians for further instruction. These patients should also be instructed to carry a warning card indicating that they may need supplementary systemic corticosteroids during periods of stress or a severe asthma attack.
Patients requiring oral corticosteroids should be weaned slowly from systemic corticosteroid use after transferring to Fluticasone Propionate and Salmeterol HFA. Prednisone reduction can be accomplished by reducing the daily prednisone dose by 2.5 mg on a weekly basis during therapy with Fluticasone Propionate and Salmeterol HFA. Lung function (mean forced expiratory volume in 1 second [FEV1] or morning peak expiratory flow [AM PEF]), beta-agonist use, and asthma symptoms should be carefully monitored during withdrawal of oral corticosteroids. In addition, patients should be observed for signs and symptoms of adrenal insufficiency, such as fatigue, lassitude, weakness, nausea and vomiting, and hypotension.
Unmasking of Allergic Conditions Previously Suppressed by Systemic Corticosteroids
Transfer of patients from systemic corticosteroid therapy to Fluticasone Propionate and Salmeterol HFA may unmask allergic conditions previously suppressed by the systemic corticosteroid therapy (e.g., rhinitis, conjunctivitis, eczema, arthritis, eosinophilic conditions).
Corticosteroid Withdrawal Symptoms
During withdrawal from oral corticosteroids, some patients may experience symptoms of systemically active corticosteroid withdrawal (e.g., joint and/or muscular pain, lassitude, depression) despite maintenance or even improvement of respiratory function.
5.8 Hypercorticism and Adrenal Suppression
Fluticasone propionate, a component of Fluticasone Propionate and Salmeterol HFA, will often help control asthma symptoms with less suppression of HPA function than therapeutically equivalent oral doses of prednisone. Since fluticasone propionate is absorbed into the circulation and can be systemically active at higher doses, the beneficial effects of Fluticasone Propionate and Salmeterol HFA in minimizing HPA dysfunction may be expected only when recommended dosages are not exceeded and individual patients are titrated to the lowest effective dose. A relationship between plasma levels of fluticasone propionate and inhibitory effects on stimulated cortisol production has been shown after 4 weeks of treatment with fluticasone propionate inhalation aerosol. Since individual sensitivity to effects on cortisol production exists, physicians should consider this information when prescribing Fluticasone Propionate and Salmeterol HFA.
Because of the possibility of significant systemic absorption of ICS in sensitive patients, patients treated with Fluticasone Propionate and Salmeterol HFA should be observed carefully for any evidence of systemic corticosteroid effects. Particular care should be taken in observing patients postoperatively or during periods of stress for evidence of inadequate adrenal response.
It is possible that systemic corticosteroid effects such as hypercorticism and adrenal suppression (including adrenal crisis) may appear in a small number of patients who are sensitive to these effects. If such effects occur, Fluticasone Propionate and Salmeterol HFA should be reduced slowly, consistent with accepted procedures for reducing systemic corticosteroids, and other treatments for management of asthma symptoms should be considered.
5.9 Drug Interactions with Strong Cytochrome P450 3A4 Inhibitors
The use of strong cytochrome P450 3A4 (CYP3A4) inhibitors (e.g., ritonavir, atazanavir, clarithromycin, indinavir, itraconazole, nefazodone, nelfinavir, saquinavir, ketoconazole, telithromycin) with Fluticasone Propionate and Salmeterol HFA is not recommended because increased systemic corticosteroid and increased cardiovascular adverse effects may occur [see Drug Interactions (7.1), Clinical Pharmacology (12.3)].
5.10 Paradoxical Bronchospasm and Upper Airway Symptoms
As with other inhaled medicines, Fluticasone Propionate and Salmeterol HFA can produce paradoxical bronchospasm, which may be life threatening. If paradoxical bronchospasm occurs following dosing with Fluticasone Propionate and Salmeterol HFA, it should be treated immediately with an inhaled, short- acting bronchodilator; Fluticasone Propionate and Salmeterol HFA should be discontinued immediately; and alternative therapy should be instituted. Upper airway symptoms of laryngeal spasm, irritation, or swelling, such as stridor and choking, have been reported in patients receiving fluticasone propionate and salmeterol HFA.
5.11 Hypersensitivity Reactions, including Anaphylaxis
Immediate hypersensitivity reactions (e.g., urticaria, angioedema, rash, bronchospasm, hypotension), including anaphylaxis, may occur after administration of Fluticasone Propionate and Salmeterol HFA [see Contraindications (4)].
5.12 Cardiovascular and Central Nervous System Effects
Excessive beta-adrenergic stimulation has been associated with seizures, angina, hypertension or hypotension, tachycardia with rates up to 200 beats/min, arrhythmias, nervousness, headache, tremor, palpitation, nausea, dizziness, fatigue, malaise, and insomnia [see Overdosage (10)]. Therefore, Fluticasone Propionate and Salmeterol HFA, like all products containing sympathomimetic amines, should be used with caution in patients with cardiovascular disorders, especially coronary insufficiency, cardiac arrhythmias, and hypertension.
Salmeterol, a component of Fluticasone Propionate and Salmeterol HFA, can produce a clinically significant cardiovascular effect in some patients as measured by pulse rate, blood pressure, and/or symptoms. Although such effects are uncommon after administration of salmeterol at recommended doses, if they occur, the drug may need to be discontinued. In addition, beta-agonists have been reported to produce electrocardiogram (ECG) changes, such as flattening of the T wave, prolongation of the QTc interval, and ST segment depression. The clinical significance of these findings is unknown. Large doses of inhaled or oral salmeterol (12 to 20 times the recommended dose) have been associated with clinically significant prolongation of the QTc interval, which has the potential for producing ventricular arrhythmias. Fatalities have been reported in association with excessive use of inhaled sympathomimetic drugs.
5.13 Reduction in Bone Mineral Density
Decreases in bone mineral density (BMD) have been observed with long-term administration of products containing ICS. The clinical significance of small changes in BMD with regard to long-term consequences such as fracture is unknown. Patients with major risk factors for decreased bone mineral content, such as prolonged immobilization, family history of osteoporosis, postmenopausal status, tobacco use, advanced age, poor nutrition, or chronic use of drugs that can reduce bone mass (e.g., anticonvulsants, oral corticosteroids), should be monitored and treated with established standards of care.
2-Year Fluticasone Propionate Trial
A 2-year trial in 160 subjects (females aged 18 to 40 years, males 18 to 50) with asthma receiving chlorofluorocarbon (CFC)-propelled fluticasone propionate inhalation aerosol 88 or 440 mcg twice daily demonstrated no statistically significant changes in BMD at any time point (24, 52, 76, and 104 weeks of double-blind treatment) as assessed by dual-energy x-ray absorptiometry at lumbar regions L1 through L4.
5.14 Effect on Growth
Orally inhaled corticosteroids may cause a reduction in growth velocity when administered to pediatric patients. Monitor the growth of pediatric patients receiving Fluticasone Propionate and Salmeterol HFA routinely (e.g., via stadiometry). To minimize the systemic effects of orally inhaled corticosteroids, including Fluticasone Propionate and Salmeterol HFA, titrate each patient’s dosage to the lowest dosage that effectively controls his/her symptoms [see Dosage and Administration (2), Use in Specific Populations (8.4)].
5.15 Glaucoma and Cataracts
Glaucoma, increased intraocular pressure, and cataracts have been reported in patients with asthma following the long-term administration of ICS, including fluticasone propionate, a component of Fluticasone Propionate and Salmeterol HFA. Consider referral to an ophthalmologist in patients who develop ocular symptoms or use Fluticasone Propionate and Salmeterol HFA long term [see Adverse Reactions (6)].
5.16 Eosinophilic Conditions and Churg-Strauss Syndrome
In rare cases, patients on inhaled fluticasone propionate, a component of Fluticasone Propionate and Salmeterol HFA, may present with systemic eosinophilic conditions. Some of these patients have clinical features of vasculitis consistent with Churg-Strauss syndrome, a condition that is often treated with systemic corticosteroid therapy. These events usually, but not always, have been associated with the reduction and/or withdrawal of oral corticosteroid therapy following the introduction of fluticasone propionate. Cases of serious eosinophilic conditions have also been reported with other ICS in this clinical setting. Physicians should be alert to eosinophilia, vasculitic rash, worsening pulmonary symptoms, cardiac complications, and/or neuropathy presenting in their patients. A causal relationship between fluticasone propionate and these underlying conditions has not been established.
5.17 Coexisting Conditions
Fluticasone Propionate and Salmeterol HFA, like all medicines containing sympathomimetic amines, should be used with caution in patients with convulsive disorders or thyrotoxicosis and in those who are unusually responsive to sympathomimetic amines. Large doses of the related beta2-adrenoceptor agonist albuterol, when administered intravenously, have been reported to aggravate preexisting diabetes mellitus and ketoacidosis.
5.18 Hypokalemia and Hyperglycemia
Beta-adrenergic agonist medicines may produce significant hypokalemia in some patients, possibly through intracellular shunting, which has the potential to produce adverse cardiovascular effects [see Clinical Pharmacology (12.2)]. The decrease in serum potassium is usually transient, not requiring supplementation. Clinically significant changes in blood glucose and/or serum potassium were seen infrequently during clinical trials with fluticasone propionate and salmeterol HFA at recommended doses.
•
LABA monotherapy increases the risk of serious asthma-related events. (5.1)
•
Do not initiate in acutely deteriorating asthma or to treat acute symptoms. (5.2)
•
Do not use in combination with an additional medicine containing a LABA because of risk of overdose. (5.3)
•
Candida albicans infection of the mouth and pharynx may occur. Monitor patients periodically. Advise the patient to rinse his/her mouth with water without swallowing after inhalation to help reduce the risk. (5.4)
•
Increased risk of pneumonia in patients with COPD. Monitor patients for signs and symptoms of pneumonia. (5.5)
•
Potential worsening of infections (e.g., existing tuberculosis; fungal, bacterial, viral, or parasitic infections; ocular herpes simplex). Use with caution in patients with these infections. More serious or even fatal course of chickenpox or measles can occur in susceptible patients. (5.6)
•
Risk of impaired adrenal function when transferring from systemic corticosteroids. Taper patients slowly from systemic corticosteroids if transferring to Fluticasone Propionate and Salmeterol HFA. (5.7)
•
Hypercorticism and adrenal suppression may occur with very high dosages or at the regular dosage in susceptible individuals. If such changes occur, discontinue Fluticasone Propionate and Salmeterol HFA slowly. (5.8)
•
If paradoxical bronchospasm occurs, discontinue Fluticasone Propionate and Salmeterol HFA and institute alternative therapy. (5.10)
•
Use with caution in patients with cardiovascular or central nervous system disorders because of beta-adrenergic stimulation. (5.12)
•
Assess for decrease in bone mineral density initially and periodically thereafter. (5.13)
•
Monitor growth of pediatric patients. (5.14)
•
Glaucoma and cataracts may occur with long-term use of inhaled corticosteroids. Consider referral to an ophthalmologist in patients who develop ocular symptoms or use Fluticasone Propionate and Salmeterol HFA long term. (5.15)
•
Be alert to eosinophilic conditions, hypokalemia, and hyperglycemia. (5.16, 5.18)
•
Use with caution in patients with convulsive disorders, thyrotoxicosis, diabetes mellitus, and ketoacidosis. (5.17)
ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
•
Serious asthma-related events – hospitalizations, intubations, death [see Warnings and Precautions (5.1)]
•
Oropharyngeal candidiasis [see Warnings and Precautions (5.4)]
•
Pneumonia in patients with COPD [see Warnings and Precautions (5.5)]
•
Immunosuppression and risk of infections [see Warnings and Precautions (5.6)]
•
Hypercorticism and adrenal suppression [see Warnings and Precautions (5.8)]
•
Cardiovascular and central nervous system effects [see Warnings and Precautions (5.12)]
•
Reduction in bone mineral density [see Warnings and Precautions (5.13)]
•
Growth effects [see Warnings and Precautions (5.14)]
•
Glaucoma and cataracts [see Warnings and Precautions (5.15)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adult and Adolescent Subjects Aged 12 Years and Older
The incidence of adverse reactions associated with fluticasone propionate and salmeterol HFA in Table 2 is based upon two 12-week, placebo-controlled, U.S. clinical trials (Trials 1 and 3) and 1 active-controlled 12-week U.S. clinical trial (Trial 2). A total of 1,008 adult and adolescent subjects with asthma (556 females and 452 males) previously treated with albuterol alone, salmeterol, or ICS were treated twice daily with 2 inhalations of fluticasone propionate and salmeterol HFA 45 mcg/21 mcg or fluticasone propionate and salmeterol HFA 115 mcg/21 mcg, fluticasone propionate CFC inhalation aerosol (44- or 110-mcg doses), salmeterol CFC inhalation aerosol 21 mcg, or placebo HFA inhalation aerosol. The average duration of exposure was 71 to 81 days in the active treatment groups compared with 51 days in the placebo group.
Table 2. Adverse Reactions with Fluticasone Propionate and Salmeterol HFA with ≥3% Incidence in Adult and Adolescent Subjects with Asthma
|
Fluticasone Propionate and Salmeterol HFA |
Fluticasone Propionate CFC Inhalation Aerosol |
Salmeterol CFC Inhalation Aerosol |
Placebo HFA Inhalation Aerosol | ||
|
|
|
|
|
| |
|
Ear, nose, and throat | ||||||
|
Upper respiratory tract infection |
16 |
24 |
13 |
15 |
17 |
13 |
|
Throat irritation |
9 |
7 |
12 |
13 |
9 |
7 |
|
Upper respiratory inflammation |
4 |
4 |
3 |
7 |
5 |
3 |
|
Hoarseness/dysphonia |
3 |
1 |
2 |
0 |
1 |
0 |
|
Lower respiratory | ||||||
|
Viral respiratory infection |
3 |
5 |
4 |
5 |
3 |
4 |
|
Neurology | ||||||
|
Headache |
21 |
15 |
24 |
16 |
20 |
11 |
|
Dizziness |
4 |
1 |
1 |
0 |
<1 |
0 |
|
Gastrointestinal | ||||||
|
Nausea and vomiting |
5 |
3 |
4 |
2 |
2 |
3 |
|
Viral gastrointestinal infection |
4 |
2 |
2 |
0 |
1 |
2 |
|
Gastrointestinal signs and symptoms |
3 |
2 |
2 |
1 |
1 |
1 |
|
Musculoskeletal | ||||||
|
Musculoskeletal pain |
5 |
7 |
8 |
2 |
4 |
4 |
|
Muscle pain |
4 |
1 |
1 |
1 |
3 |
<1 |
The incidence of common adverse reactions reported in Trial 4, a 12-week non-U.S. clinical trial in 509 subjects previously treated with ICS who were treated twice daily with 2 inhalations of fluticasone propionate and salmeterol HFA 230 mcg/21 mcg, fluticasone propionate CFC inhalation aerosol 220 mcg, or 1 inhalation of ADVAIR DISKUS 500 mcg/50 mcg was similar to the incidences reported in Table 2.
Additional Adverse Reactions
Other adverse reactions not previously listed, whether considered drug-related or not by the investigators, that occurred in the groups receiving fluticasone propionate and salmeterol HFA with an incidence of 1% to 3% and that occurred at a greater incidence than with placebo include the following: tachycardia, arrhythmias, myocardial infarction, postoperative complications, wounds and lacerations, soft tissue injuries, ear signs and symptoms, rhinorrhea/postnasal drip, epistaxis, nasal congestion/blockage, laryngitis, unspecified oropharyngeal plaques, dryness of nose, weight gain, allergic eye disorders, eye edema and swelling, gastrointestinal discomfort and pain, dental discomfort and pain, candidiasis mouth/throat, hyposalivation, gastrointestinal infections, disorders of hard tissue of teeth, abdominal discomfort and pain, oral abnormalities, arthralgia and articular rheumatism, muscle cramps and spasms, musculoskeletal inflammation, bone and skeletal pain, muscle injuries, sleep disorders, migraines, allergies and allergic reactions, viral infections, bacterial infections, candidiasis unspecified site, congestion, inflammation, bacterial reproductive infections, lower respiratory signs and symptoms, lower respiratory infections, lower respiratory hemorrhage, eczema, dermatitis and dermatosis, urinary infections.
Laboratory Test Abnormalities
In Trial 3, there were more reports of hyperglycemia among adults and adolescents receiving fluticasone propionate and salmeterol HFA, but this was not seen in Trials 1 and 2.
6.2 Postmarketing Experience
In addition to adverse reactions reported from clinical trials, the following adverse reactions have been identified during postapproval use of any formulation of fluticasone propionate and salmeterol, fluticasone propionate, and/or salmeterol regardless of indication. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. These events have been chosen for inclusion due to either their seriousness, frequency of reporting, or causal connection to fluticasone propionate and salmeterol, fluticasone propionate, and/or salmeterol or a combination of these factors.
Cardiovascular
Arrhythmias (including atrial fibrillation, extrasystoles, supraventricular tachycardia), hypertension, ventricular tachycardia.
Ear, Nose, and Throat
Aphonia, earache, facial and oropharyngeal edema, paranasal sinus pain, rhinitis, throat soreness, tonsillitis.
Endocrine and Metabolic
Cushing’s syndrome, Cushingoid features, growth velocity reduction in children/adolescents, hypercorticism, osteoporosis.
Eye
Cataracts, glaucoma.
Gastrointestinal
Dyspepsia, xerostomia.
Hepatobiliary Tract and Pancreas
Abnormal liver function tests.
Immune System
Immediate and delayed hypersensitivity reactions, including rash and rare events of angioedema, bronchospasm, and anaphylaxis.
Infections and Infestations
Esophageal candidiasis.
Musculoskeletal
Back pain, myositis.
Neurology
Paresthesia, restlessness.
Non-Site Specific
Fever, pallor.
Psychiatry
Agitation, aggression, anxiety, depression. Behavioral changes, including hyperactivity and irritability, have been reported very rarely and primarily in children.
Respiratory
Asthma; asthma exacerbation; chest congestion; chest tightness; cough; dyspnea; immediate bronchospasm; influenza; paradoxical bronchospasm; tracheitis; wheezing; pneumonia; reports of upper respiratory symptoms of laryngeal spasm, irritation, or swelling such as stridor or choking.
Skin
Contact dermatitis, contusions, ecchymoses, photodermatitis, pruritus.
Urogenital
Dysmenorrhea, irregular menstrual cycle, pelvic inflammatory disease, vaginal candidiasis, vaginitis, vulvovaginitis.
Most common adverse reactions (incidence ≥3%) include: upper respiratory tract infection or inflammation, throat irritation, dysphonia, headache, dizziness, nausea and vomiting. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Prasco Laboratories at 1-866-525-0688 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
Fluticasone propionate and salmeterol HFA has been studied in subjects with asthma aged 12 years and older. Fluticasone propionate and salmeterol HFA has not been studied in subjects younger than 12 years or in subjects with COPD. In clinical trials comparing fluticasone propionate and salmeterol HFA with its individual components, improvements in most efficacy endpoints were greater with fluticasone propionate and salmeterol HFA than with the use of either fluticasone propionate or salmeterol alone. In addition, clinical trials showed comparable results between fluticasone propionate and salmeterol HFA and ADVAIR DISKUS.
14.1 Trials Comparing Fluticasone Propionate and Salmeterol HFA with
Fluticasone Propionate Alone or Salmeterol Alone
Four (4) double-blind, parallel-group clinical trials were conducted with fluticasone propionate and salmeterol HFA in 1,517 adult and adolescent subjects (aged 12 years and older, mean baseline FEV1 65% to 75% of predicted normal) with asthma that was not optimally controlled on their current therapy. All metered-dose inhaler treatments were inhalation aerosols given as 2 inhalations twice daily, and other maintenance therapies were discontinued.
Trial 1: Clinical Trial with Fluticasone Propionate and Salmeterol HFA 45 mcg/21 mcg
This placebo-controlled, 12-week, U.S. trial compared fluticasone propionate and salmeterol HFA 45 mcg/21 mcg with fluticasone propionate CFC inhalation aerosol 44 mcg or salmeterol CFC inhalation aerosol 21 mcg, each given as 2 inhalations twice daily. The primary efficacy endpoints were predose FEV1 and withdrawals due to worsening asthma. This trial was stratified according to baseline asthma therapy: subjects using beta-agonists (albuterol alone [n = 142], salmeterol [n = 84], or ICS [n = 134] [daily doses of beclomethasone dipropionate 252 to 336 mcg; budesonide 400 to 600 mcg; flunisolide 1,000 mcg; fluticasone propionate inhalation aerosol 176 mcg; fluticasone propionate inhalation powder 200 mcg; or triamcinolone acetonide 600 to 800 mcg]). Baseline FEV1 measurements were similar across treatments: fluticasone propionate and salmeterol HFA 45 mcg/21 mcg, 2.29 L; fluticasone propionate 44 mcg, 2.20 L; salmeterol, 2.33 L; and placebo, 2.27 L.
Predefined withdrawal criteria for lack of efficacy, an indicator of worsening asthma, were utilized for this placebo-controlled trial. Worsening asthma was defined as a clinically important decrease in FEV1 or PEF, increase in use of VENTOLIN (albuterol, USP) Inhalation Aerosol, increase in night awakenings due to asthma, emergency intervention or hospitalization due to asthma, or requirement for asthma medicine not allowed by the protocol. As shown in Table 3, statistically significantly fewer subjects receiving fluticasone propionate and salmeterol HFA 45 mcg/21 mcg were withdrawn due to worsening asthma compared with salmeterol and placebo. Fewer subjects receiving fluticasone propionate and salmeterol HFA 45 mcg/21 mcg were withdrawn due to worsening asthma compared with fluticasone propionate 44 mcg; however, the difference was not statistically significant.
Table 3. Percent of Subjects Withdrawn due to Worsening Asthma in Subjects Previously Treated with Beta2-agonists (Albuterol or Salmeterol) or Inhaled Corticosteroids (Trial 1)|
Fluticasone Propionate and Salmeterol HFA 45 mcg/21 mcg (n = 92) |
Fluticasone Propionate CFC Inhalation Aerosol 44 mcg (n = 89) |
Salmeterol CFC Inhalation Aerosol 21 mcg (n = 92) |
Placebo HFA Inhalation Aerosol (n = 87) |
|
2% |
8% |
25% |
28% |
The FEV1 results are displayed in Figure 1. Because this trial used predetermined criteria for worsening asthma, which caused more subjects in the placebo group to be withdrawn, FEV1 results at Endpoint (last available FEV1 result) are also provided. Subjects receiving fluticasone propionate and salmeterol HFA 45 mcg/21 mcg had significantly greater improvements in FEV1 (0.58 L, 27%) compared with fluticasone propionate 44 mcg (0.36 L, 18%), salmeterol (0.25 L, 12%), and placebo (0.14 L, 5%). These improvements in FEV1 with fluticasone propionate and salmeterol HFA 45 mcg/21 mcg were achieved regardless of baseline asthma therapy (albuterol alone, salmeterol, or ICS).

Figure 1. Mean Percent Change from Baseline in FEV1 in Subjects Previously Treated with Either Beta2-agonists (Albuterol or Salmeterol) or Inhaled Corticosteroids (Trial 1)
The effect of fluticasone propionate and salmeterol HFA 45 mcg/21 mcg on the secondary efficacy parameters, including morning and evening PEF, usage of VENTOLIN Inhalation Aerosol, and asthma symptoms over 24 hours on a scale of 0 to 5 is shown in Table 4.
Table 4. Secondary Efficacy Variable Results for Subjects Previously Treated with Beta2agonists (Albuterol or Salmeterol) or Inhaled Corticosteroids (Trial 1)|
a Change from baseline = change from baseline at Endpoint (last available data). | ||||
|
Efficacy Variable****a |
Fluticasone Propionate and Salmeterol HFA 45 mcg/21 mcg (n = 92) |
Fluticasone Propionate CFC Inhalation Aerosol 44 mcg (n = 89) |
Salmeterol CFC Inhalation Aerosol 21 mcg (n = 92) |
Placebo HFA Inhalation Aerosol (n = 87) |
|
AM PEF (L/min) | ||||
|
377 |
369 |
381 |
382 |
|
58 |
27 |
25 |
1 |
|
PM PEF (L/min) | ||||
|
397 |
387 |
402 |
407 |
|
48 |
20 |
16 |
3 |
|
Use of VENTOLIN Inhalation Aerosol (inhalations/day) | ||||
|
3.1 |
2.4 |
2.7 |
2.7 |
|
-2.1 |
-0.4 |
-0.8 |
0.2 |
|
Asthma symptom score/day | ||||
|
1.8 |
1.6 |
1.7 |
1.7 |
|
-1.0 |
-0.3 |
-0.4 |
0 |
The subjective impact of asthma on subjects’ perception of health was evaluated through use of an instrument called the Asthma Quality of Life Questionnaire (AQLQ) (based on a 7-point scale where 1 = maximum impairment and 7 = none). Subjects receiving fluticasone propionate and salmeterol HFA 45 mcg/21 mcg had clinically meaningful improvements in overall asthma-specific quality of life as defined by a difference between groups of ≥0.5 points in change from baseline AQLQ scores (difference in AQLQ score of 1.14 [95% CI: 0.85, 1.44] compared with placebo).
Trial 2: Clinical Trial with Fluticasone Propionate and Salmeterol HFA 45 mcg/21 mcg
This active-controlled, 12-week, U.S. trial compared fluticasone propionate and salmeterol HFA 45 mcg/21 mcg with fluticasone propionate CFC inhalation aerosol 44 mcg and salmeterol CFC inhalation aerosol 21 mcg, each given as 2 inhalations twice daily, in 283 subjects using as-needed albuterol alone. The primary efficacy endpoint was predose FEV1. Baseline FEV1 measurements were similar across treatments: fluticasone propionate and salmeterol HFA 45 mcg/21 mcg, 2.37 L; fluticasone propionate 44 mcg, 2.31 L; and salmeterol, 2.34 L.
Efficacy results in this trial were similar to those observed in Trial 1. Subjects receiving fluticasone propionate and salmeterol HFA 45 mcg/21 mcg had significantly greater improvements in FEV1 (0.69 L, 33%) compared with fluticasone propionate 44 mcg (0.51 L, 25%) and salmeterol (0.47 L, 22%).
Trial 3: Clinical Trial with Fluticasone Propionate and Salmeterol HFA 115 mcg/21 mcg
This placebo-controlled, 12-week, U.S. trial compared fluticasone propionate and salmeterol HFA 115 mcg/21 mcg with fluticasone propionate CFC inhalation aerosol 110 mcg or salmeterol CFC inhalation aerosol 21 mcg, each given as 2 inhalations twice daily, in 365 subjects using ICS (daily doses of beclomethasone dipropionate 378 to 840 mcg; budesonide 800 to 1,200 mcg; flunisolide 1,250 to 2,000 mcg; fluticasone propionate inhalation aerosol 440 to 660 mcg; fluticasone propionate inhalation powder 400 to 600 mcg; or triamcinolone acetonide 900 to 1,600 mcg). The primary efficacy endpoints were predose FEV1 and withdrawals due to worsening asthma. Baseline FEV1 measurements were similar across treatments: fluticasone propionate and salmeterol HFA 115 mcg/21 mcg, 2.23 L; fluticasone propionate 110 mcg, 2.18 L; salmeterol, 2.22 L; and placebo, 2.17 L.
Efficacy results in this trial were similar to those observed in Trials 1 and 2. Subjects receiving fluticasone propionate and salmeterol HFA 115 mcg/21 mcg had significantly greater improvements in FEV1 (0.41 L, 20%) compared with fluticasone propionate 110 mcg (0.19 L, 9%), salmeterol (0.15 L, 8%), and placebo (-0.12 L, -6%). Significantly fewer subjects receiving fluticasone propionate and salmeterol HFA 115 mcg/21 mcg were withdrawn from this trial for worsening asthma (7%) compared with salmeterol (24%) and placebo (54%). Fewer subjects receiving fluticasone propionate and salmeterol HFA 115 mcg/21 mcg were withdrawn due to worsening asthma (7%) compared with fluticasone propionate 110 mcg (11%); however, the difference was not statistically significant.
Trial 4: Clinical Trial with Fluticasone Propionate and Salmeterol HFA 230 mcg/21 mcg
This active-controlled, 12-week, non-U.S. trial compared fluticasone propionate and salmeterol HFA 230 mcg/21 mcg with fluticasone propionate CFC inhalation aerosol 220 mcg, each given as 2 inhalations twice daily, and with ADVAIR DISKUS 500 mcg/50 mcg given as 1 inhalation twice daily in 509 subjects using ICS (daily doses of beclomethasone dipropionate CFC inhalation aerosol 1,500 to 2,000 mcg; budesonide 1,500 to 2,000 mcg; flunisolide 1,500 to 2,000 mcg; fluticasone propionate inhalation aerosol 660 to 880 mcg; or fluticasone propionate inhalation powder 750 to 1,000 mcg). The primary efficacy endpoint was morning PEF.
Baseline morning PEF measurements were similar across treatments: fluticasone propionate and salmeterol HFA 230 mcg/21 mcg, 327 L/min; ADVAIR DISKUS 500 mcg/50 mcg, 341 L/min; and fluticasone propionate 220 mcg, 345 L/min. As shown in Figure 2, morning PEF improved significantly with fluticasone propionate and salmeterol HFA 230 mcg/21 mcg compared with fluticasone propionate 220 mcg over the 12-week treatment period. Improvements in morning PEF observed with fluticasone propionate and salmeterol HFA 230 mcg/21 mcg were similar to improvements observed with ADVAIR DISKUS 500 mcg/50 mcg.
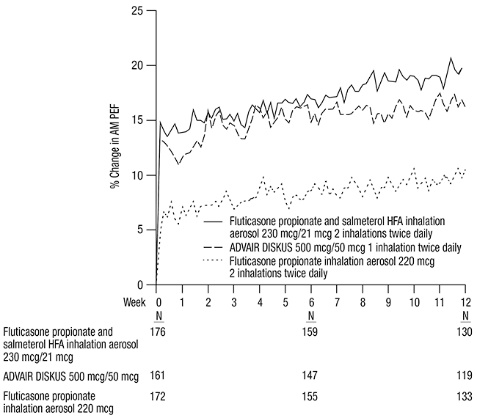
Figure 2. Mean Percent Change from Baseline in Morning Peak Expiratory Flow in Subjects Previously Treated with Inhaled Corticosteroids (Trial 4)
14.2 One-Year Safety Trial
Clinical Trial with Fluticasone Propionate and Salmeterol HFA 45 mcg/21 mcg, Fluticasone Propionate and Salmeterol HFA 115 mcg/21 mcg, and Fluticasone Propionate and Salmeterol HFA 230 mcg/21 mcg
This 1-year, open-label, non-U.S. trial evaluated the safety of fluticasone propionate and salmeterol HFA 45 mcg/21 mcg, fluticasone propionate and salmeterol HFA 115 mcg/21 mcg, and fluticasone propionate and salmeterol HFA 230 mcg/21 mcg given as 2 inhalations twice daily in 325 subjects. This trial was stratified into 3 groups according to baseline asthma therapy: subjects using short-acting beta2-agonists alone (n = 42), salmeterol (n = 91), or ICS (n = 277). Subjects treated with short-acting beta2-agonists alone, salmeterol, or low doses of ICS with or without concurrent salmeterol received fluticasone propionate and salmeterol HFA 45 mcg/21 mcg. Subjects treated with moderate doses of ICS with or without concurrent salmeterol received fluticasone propionate and salmeterol HFA 115 mcg/21 mcg. Subjects treated with high doses of ICS with or without concurrent salmeterol received fluticasone propionate and salmeterol HFA 230 mcg/21 mcg. Baseline FEV1 measurements ranged from 2.3 to 2.6 L.
Improvements in FEV1 (0.17 to 0.35 L at 4 weeks) were seen across all 3 treatments and were sustained throughout the 52-week treatment period. Few subjects (3%) were withdrawn due to worsening asthma over 1 year.
14.3 Onset of Action and Progression of Improvement in Control
The onset of action and progression of improvement in asthma control were evaluated in 2 placebo-controlled U.S. trials and 1 active-controlled U.S. trial. Following the first dose, the median time to onset of clinically significant bronchodilatation (≥15% improvement in FEV1) in most subjects was seen within 30 to 60 minutes. Maximum improvement in FEV1 occurred within 4 hours, and clinically significant improvement was maintained for 12 hours (Figure 3).
Following the initial dose, predose FEV1 relative to Day 1 baseline improved markedly over the first week of treatment and continued to improve over the 12 weeks of treatment in all 3 trials.
No diminution in the 12-hour bronchodilator effect was observed with either fluticasone propionate and salmeterol HFA 45 mcg/21 mcg (Figures 3 and 4) or fluticasone propionate and salmeterol HFA 230/21 as assessed by FEV1 following 12 weeks of therapy.
Figure 3. Percent Change in Serial 12-Hour FEV1 in Subjects Previously Using Either Beta2-agonists (Albuterol or Salmeterol) or Inhaled Corticosteroids (Trial 1)
First Treatment Day

Figure 4. Percent Change in Serial 12-Hour FEV1 in Subjects Previously Using Either Beta2-agonists (Albuterol or Salmeterol) or Inhaled Corticosteroids (Trial 1)
Last Treatment Day (Week 12)

Reduction in asthma symptoms and use of rescue VENTOLIN Inhalation Aerosol and improvement in morning and evening PEF also occurred within the first day of treatment with fluticasone propionate and salmeterol HFA and continued to improve over the 12 weeks of therapy in all 3 trials.
SPL PATIENT PACKAGE INSERT SECTION
|
PATIENT INFORMATION Fluticasone Propionate and Salmeterol HFA inhalation aerosol for oral inhalation use | |
|
What is Fluticasone Propionate and Salmeterol HFA? • o o • • • o o o | |
|
Do not use Fluticasone Propionate and Salmeterol HFA: • • • | |
|
Before using Fluticasone Propionate and Salmeterol HFA, tell your healthcare provider about all of your medical conditions, including if you: • • • • • • • • • • • • • Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Fluticasone Propionate and Salmeterol HFA and certain other medicines may interact with each other. This may cause serious side effects. Especially tell your healthcare provider if you take antifungal or anti-HIV medicines. Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine. | |
|
How should I use Fluticasone Propionate and Salmeterol HFA? Read the step-by-step instructions for using Fluticasone Propionate and Salmeterol HFA at the end of this Patient Information. • • • • • • • • • • • o o o o o o o | |
|
What are the possible side effects of Fluticasone Propionate and Salmeterol HFA? Fluticasone Propionate and Salmeterol HFA can cause serious side effects, including: • • | |
|
|
|
• • | |
|
|
|
• • | |
|
|
|
• | |
|
|
|
• | |
|
|
|
• • • • Common side effects of Fluticasone Propionate and Salmeterol HFA include: | |
|
o o o |
• • • |
|
These are not all the possible side effects of Fluticasone Propionate and Salmeterol HFA. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. | |
|
How should I store Fluticasone Propionate and Salmeterol HFA? • • • • Keep Fluticasone Propionate and Salmeterol HFA and all medicines out of the reach of children. | |
|
General information about the safe and effective use of Fluticasone Propionate and Salmeterol HFA. Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Fluticasone Propionate and Salmeterol HFA for a condition for which it was not prescribed. Do not give Fluticasone Propionate and Salmeterol HFA to other people, even if they have the same symptoms that you have. It may harm them. You can ask your healthcare provider or pharmacist for information about Fluticasone Propionate and Salmeterol HFA that was written for health professionals. | |
|
What are the ingredients in Fluticasone Propionate and Salmeterol HFA? Active ingredients: fluticasone propionate, salmeterol xinafoate Inactive ingredient: propellant HFA-134a For more information about Fluticasone Propionate and Salmeterol HFA, call 1-866-525-0688. Trademarks are owned by or licensed to the GSK group of companies. Manufactured for: Prasco Laboratories Mason, OH 45040 USA Manufactured by: GlaxoSmithKline Durham, NC 27701 ADH-PS:1PIL |
This Patient Information has been approved by the U.S. Food and Drug Administration Revised: August 2022
INSTRUCTIONS FOR USE SECTION
|
INSTRUCTIONS FOR USE Fluticasone Propionate and Salmeterol HFA inhalation aerosol for oral inhalation use | |
|
Your Fluticasone Propionate and Salmeterol HFA inhaler | |
|
Figure A |
• • |
|
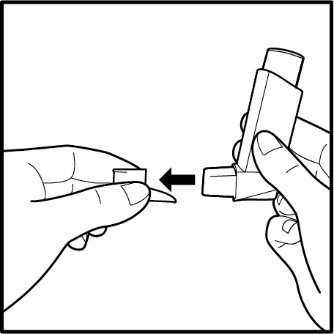
• • • • • Before using your Fluticasone Propionate and Salmeterol HFA inhaler • Priming your Fluticasone Propionate and Salmeterol HFA inhaler | |
|
Figure B
Figure C
Figure D |
• • • • • |
|
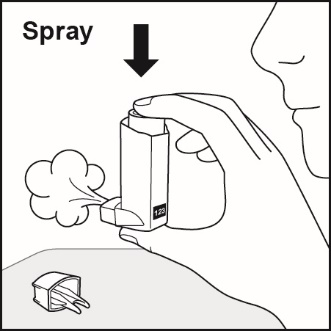
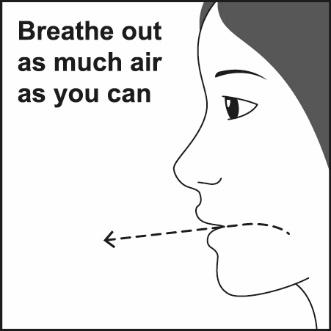
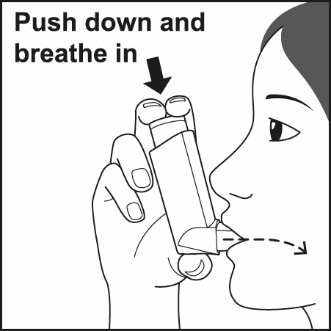
How to use your Fluticasone Propionate and Salmeterol HFA inhaler Follow these steps every time you use Fluticasone Propionate and Salmeterol HFA. | |
|
Figure E
Figure F
Figure G
Figure H |
|
| |
|
Figure I
Figure J |
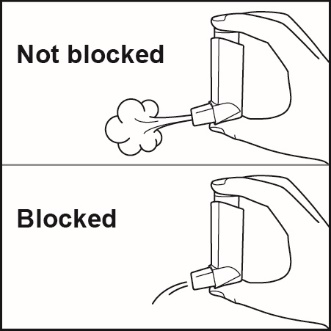
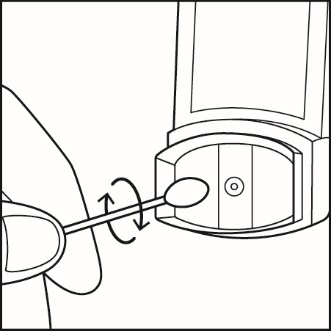
Clean your inhaler at least 1 time each week after your evening dose. You may not see any medicine build-up on the inhaler, but it is important to keep it clean so medicine build-up will not block the spray.See Figure I. |
|
Replacing your Fluticasone Propionate and Salmeterol HFA inhaler • • • For correct use of your Fluticasone Propionate and Salmeterol HFA inhaler, remember: • • • • • • • • For more information about Fluticasone Propionate and Salmeterol HFA or how to use your inhaler, call 1-866-525-0688. Manufactured for: Prasco Laboratories Mason, OH 45040 USA Manufactured by: GlaxoSmithKline Durham, NC 27701 ADH-PS:1IFU | |
|
DOSAGE FORMS & STRENGTHS SECTION
3 DOSAGE FORMS AND STRENGTHS
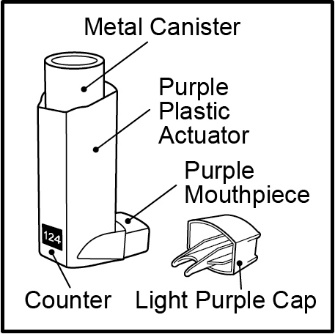
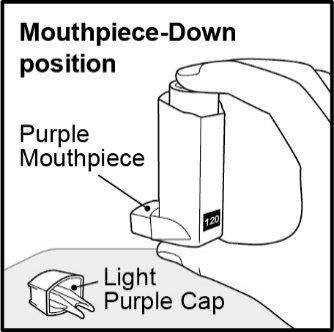
Inhalation aerosol: purple plastic inhaler with a light purple cap containing a pressurized metered-dose aerosol canister containing 120 metered inhalations and fitted with a counter.
•
45 mcg fluticasone propionate/21 mcg salmeterol from the mouthpiece per actuation
•
115 mcg fluticasone propionate/21 mcg salmeterol from the mouthpiece per actuation
•
230 mcg fluticasone propionate/21 mcg salmeterol from the mouthpiece per actuation
Inhalation aerosol:
•
45 mcg fluticasone propionate/21 mcg salmeterol per actuation (3)
•
115 mcg fluticasone propionate/21 mcg salmeterol per actuation (3)
•
230 mcg fluticasone propionate/21 mcg salmeterol per actuation (3)
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Serious Asthma-Related Events
Inform patients with asthma that LABA when used alone increases the risk of asthma-related hospitalization or asthma-related death. Available data show that when ICS and LABA are used together, such as with Fluticasone Propionate and Salmeterol HFA, there is not a significant increase in the risk of these events. [See Warnings and Precautions (5.1).]
Not for Acute Symptoms
Inform patients that Fluticasone Propionate and Salmeterol HFA is not meant to relieve acute asthma symptoms and extra doses should not be used for that purpose. Advise patients to treat acute asthma symptoms with an inhaled, short-acting beta2-agonist such as albuterol. Provide patients with such medication and instruct them in how it should be used.
Instruct patients to seek medical attention immediately if they experience any of the following:
•
Decreasing effectiveness of inhaled, short-acting beta2-agonists
•
Need for more inhalations than usual of inhaled, short-acting beta2-agonists
•
Significant decrease in lung function as outlined by the physician
Tell patients they should not stop therapy with Fluticasone Propionate and Salmeterol HFA without physician/provider guidance since symptoms may recur after discontinuation. [See Warnings and Precautions (5.2).]
Do Not Use Additional Long-acting Beta2-agonists
Instruct patients not to use other LABA for asthma. [See Warnings and Precautions (5.3).]
Oropharyngeal Candidiasis
Inform patients that localized infections with Candida albicans occurred in the mouth and pharynx in some patients. If oropharyngeal candidiasis develops, treat it with appropriate local or systemic (i.e., oral) antifungal therapy while still continuing therapy with Fluticasone Propionate and Salmeterol HFA, but at times therapy with Fluticasone Propionate and Salmeterol HFA may need to be temporarily interrupted under close medical supervision. Advise patients to rinse the mouth with water without swallowing after inhalation to help reduce the risk of thrush. [See Warnings and Precautions (5.4).]
Pneumonia
Patients with COPD have a higher risk of pneumonia; instruct them to contact their healthcare providers if they develop symptoms of pneumonia. [See Warnings and Precautions (5.5).]
Immunosuppression and Risk of Infections
Warn patients who are on immunosuppressant doses of corticosteroids to avoid exposure to chickenpox or measles and, if exposed, to consult their physicians without delay. Inform patients of potential worsening of existing tuberculosis; fungal, bacterial, viral, or parasitic infections; or ocular herpes simplex. [See Warnings and Precautions (5.6).]
Hypercorticism and Adrenal Suppression
Advise patients that Fluticasone Propionate and Salmeterol HFA may cause systemic corticosteroid effects of hypercorticism and adrenal suppression. Additionally, inform patients that deaths due to adrenal insufficiency have occurred during and after transfer from systemic corticosteroids. Patients should taper slowly from systemic corticosteroids if transferring to Fluticasone Propionate and Salmeterol HFA. [See Warnings and Precautions (5.8).]
Hypersensitivity Reactions, including Anaphylaxis
Advise patients that immediate hypersensitivity reactions (e.g., urticaria, angioedema, rash, bronchospasm, hypotension), including anaphylaxis, may occur after administration of Fluticasone Propionate and Salmeterol HFA. Patients should discontinue Fluticasone Propionate and Salmeterol HFA if such reactions occur. [See Warnings and Precautions (5.11).]
Risks Associated with Beta-agonist Therapy
Inform patients of adverse effects associated with beta2-agonists, such as palpitations, chest pain, rapid heart rate, tremor, or nervousness. [See Warnings and Precautions (5.12).]
Reduction in Bone Mineral Density
Advise patients who are at an increased risk for decreased BMD that the use of corticosteroids may pose an additional risk. [See Warnings and Precautions (5.13).]
Reduced Growth Velocity
Inform patients that orally inhaled corticosteroids, including fluticasone propionate, may cause a reduction in growth velocity when administered to pediatric patients. Physicians should closely follow the growth of children and adolescents taking corticosteroids by any route. [See Warnings and Precautions (5.14).]
Glaucoma and Cataracts
Advise patients that long-term use of ICS may increase the risk of some eye problems (cataracts or glaucoma); consider regular eye examinations. [See Warnings and Precautions (5.15).]
Trademarks are owned by or licensed to the GSK group of companies.
Manufactured for:
Prasco Laboratories
Mason, OH 45040 USA
Manufactured by:
GlaxoSmithKline
Durham, NC 27701
ADH-PS:2PI
DESCRIPTION SECTION
11 DESCRIPTION
Fluticasone Propionate and Salmeterol HFA 45 mcg/21 mcg inhalation aerosol, Fluticasone Propionate and Salmeterol HFA inhalation aerosol 115 mcg/21 mcg, and Fluticasone Propionate and Salmeterol HFA inhalation aerosol 230 mcg/21 mcg are combinations of fluticasone propionate and salmeterol xinafoate.
One active component of Fluticasone Propionate and Salmeterol HFA is fluticasone propionate, a corticosteroid having the chemical name S-(fluoromethyl) 6α,9-difluoro-11β,17-dihydroxy-16α-methyl-3-oxoandrosta-1,4-diene-17β-carbothioate, 17-propionate and the following chemical structure:

Fluticasone propionate is a white powder with a molecular weight of 500.6, and the empirical formula is C25H31F3O5S. It is practically insoluble in water, freely soluble in dimethyl sulfoxide and dimethylformamide, and slightly soluble in methanol and 95% ethanol.
The other active component of Fluticasone Propionate and Salmeterol HFA is salmeterol xinafoate, a beta2-adrenergic bronchodilator. Salmeterol xinafoate is the racemic form of the 1-hydroxy-2-naphthoic acid salt of salmeterol. It has the chemical name 4-hydroxy-α1-[[[6-(4-phenylbutoxy)hexyl]amino]methyl]-1,3-benzenedimethanol, 1-hydroxy-2-naphthalenecarboxylate and the following chemical structure:

Salmeterol xinafoate is a white powder with a molecular weight of 603.8, and the empirical formula is C25H37NO4•C11H8O3. It is freely soluble in methanol; slightly soluble in ethanol, chloroform, and isopropanol; and sparingly soluble in water.
Fluticasone Propionate and Salmeterol HFA is a purple plastic inhaler with a light purple cap containing a pressurized metered-dose aerosol canister fitted with a counter. Each canister contains a microcrystalline suspension of micronized fluticasone propionate and micronized salmeterol xinafoate in propellant HFA-134a (1,1,1,2-tetrafluoroethane). It contains no other excipients.
After priming, each actuation of the inhaler delivers 50, 125, or 250 mcg of fluticasone propionate and 25 mcg of salmeterol in 75 mg of suspension from the valve. Each actuation delivers 45, 115, or 230 mcg of fluticasone propionate and 21 mcg of salmeterol from the actuator. Twenty-one micrograms (21 mcg) of salmeterol base is equivalent to 30.45 mcg of salmeterol xinafoate. The actual amount of drug delivered to the lung will depend on patient factors, such as the coordination between the actuation of the inhaler and inspiration through the delivery system.
Prime Fluticasone Propionate and Salmeterol HFA before using for the first time by releasing 4 sprays into the air away from the face, shaking well for 5 seconds before each spray. In cases where the inhaler has not been used for more than 4 weeks or when it has been dropped, prime the inhaler again by releasing 2 sprays into the air away from the face, shaking well for 5 seconds before each spray. Avoid spraying in eyes.