LEVEMIR
These highlights do not include all the information needed to use LEVEMIR safely and effectively.See full prescribing information for LEVEMIR. LEVEMIR (insulin detemir) injection, for subcutaneous useInitial U.S. Approval: 2005
35e31793-9df4-4773-82b2-59678d8652bf
HUMAN PRESCRIPTION DRUG LABEL
Mar 12, 2023
A-S Medication Solutions
DUNS: 830016429
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
insulin detemir
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (10)
Drug Labeling Information
WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Never Share a LEVEMIR FlexPen, Needle, or Insulin Syringe between
Patients
LEVEMIR FlexPen prefilled pens must never be shared between patients, even if the needle is changed. Patients using LEVEMIR vials should never share needles or syringes with another person. Sharing poses a risk for transmission of blood-borne pathogens.
5.2 Hyperglycemia or Hypoglycemia with Changes in Insulin Regimen
Changes in an insulin regimen (e.g., insulin strength, manufacturer, type, injection site or method of administration) may affect glycemic control and predispose to hypoglycemia [see Warnings and Precautions (5.4)] or hyperglycemia. Repeated insulin injections into areas of lipodystrophy or localized cutaneous amyloidosis have been reported to result in hyperglycemia; and a sudden change in the injection site (to an unaffected area) has been reported to result in hypoglycemia [see Adverse Reactions (6)].
Make any changes to a patient’s insulin regimen under close medical supervision with increased frequency of blood glucose monitoring. Advise patients who have repeatedly injected into areas of lipodystrophy or localized cutaneous amyloidosis to change the injection site to unaffected areas and closely monitor for hypoglycemia. For patients with type 2 diabetes, dosage adjustments of concomitant antidiabetic products may be needed [see Dosage and Administration (2.4)].
5.3 Hypoglycemia
Hypoglycemia is the most common adverse reaction of insulin, including LEVEMIR [see Adverse Reactions (6.1)]. Severe hypoglycemia can cause seizures, may be life-threatening or cause death. Hypoglycemia can impair concentration ability and reaction time; this may place the patient and others at risk in situations where these abilities are important (e.g., driving or operating other machinery). LEVEMIR, or any insulin, should not be used during episodes of hypoglycemia [see Contraindications (4)].
Hypoglycemia can happen suddenly and symptoms may differ in each patient and change over time in the same patient. Symptomatic awareness of hypoglycemia may be less pronounced in patients with longstanding diabetes, in patients with diabetic neuropathy, using drugs that block the sympathetic nervous system (e.g., beta-blockers) [see Drug Interactions (7)], or who experience recurrent hypoglycemia.
Risk Factors for Hypoglycemia
The risk of hypoglycemia generally increases with intensity of glycemic control. The risk of hypoglycemia after an injection is related to the duration of action of the insulin [see Clinical Pharmacology (12.2)] and, in general, is highest when the glucose lowering effect of the insulin is maximal. As with all insulins, the glucose lowering effect time course of LEVEMIR may vary among different patients or at different times in the same patient and depends on many conditions, including the area of injection as well as the injection site blood supply and temperature.
Other factors which may increase the risk of hypoglycemia include changes in meal pattern (e.g., macronutrient content or timing of meals), changes in level of physical activity, or changes to concomitant drugs [see Drug Interactions (7)]. When a GLP-1 receptor agonist is used in combination with LEVEMIR, the LEVEMIR dose may need to be lowered or more conservatively titrated to minimize the risk of hypoglycemia [see Adverse Reactions (6.1)]. Patients with renal or hepatic impairment may be at higher risk of hypoglycemia [see Use in Specific Populations (8.6, 8.7)].
Risk Mitigation Strategies for Hypoglycemia
Patients and caregivers must be educated to recognize and manage hypoglycemia. Self-monitoring of blood glucose plays an essential role in the prevention and management of hypoglycemia. In patients at higher risk for hypoglycemia and patients who have reduced symptomatic awareness of hypoglycemia, increased frequency of blood glucose monitoring is recommended.
5.4 Hypoglycemia Due to Medication Errors
Accidental mix-ups between insulin products have been reported. To avoid medication errors between LEVEMIR and other insulins, instruct patients to always check the insulin label before each injection.
5.5 Hypersensitivity Reactions
Severe, life-threatening, generalized allergy, including anaphylaxis, can occur with insulins, including LEVEMIR [see Adverse Reactions (6.1)]. If hypersensitivity reactions occur, discontinue LEVEMIR; treat per standard of care and monitor until symptoms and signs resolve. LEVEMIR is contraindicated in patients who have had hypersensitivity reactions to insulin detemir or any of the excipients.
5.6 Hypokalemia
All insulins, including LEVEMIR, cause a shift in potassium from the extracellular to intracellular space, possibly leading to hypokalemia. Untreated hypokalemia may cause respiratory paralysis, ventricular arrhythmia, and death. Monitor potassium levels in patients at risk for hypokalemia if indicated (e.g., patients using potassium-lowering medications, patients taking medications sensitive to serum potassium concentrations).
5.7 Fluid Retention and Heart Failure with Concomitant Use of PPAR-gamma
Agonists
Thiazolidinediones (TZDs), which are peroxisome proliferator-activated receptor (PPAR)-gamma agonists, can cause dose-related fluid retention, when used in combination with insulin. Fluid retention may lead to or exacerbate heart failure. Patients treated with insulin, including LEVEMIR, and a PPAR- gamma agonist should be observed for signs and symptoms of heart failure. If heart failure develops, it should be managed according to current standards of care, and discontinuation or dose reduction of the PPAR-gamma agonist must be considered.
•
Never Share a LEVEMIR FlexPen, insulin syringe, or needle between patients, even if the needle is changed (5.1).
•
Hyperglycemia or hypoglycemia with changes in insulin regimen: Make changes to a patient’s insulin regimen (e.g., insulin strength, manufacturer, type, injection site or method of administration) under close medical supervision with increased frequency of blood glucose monitoring (5.2).
•
Hypoglycemia: May be life-threatening. Increase frequency of glucose monitoring with changes to: insulin dosage, concomitant drugs, meal pattern, physical activity; and in patients with renal impairment or hepatic impairment or hypoglycemia unawareness (5.3).
•
Hypoglycemia due to medication errors: Accidental mix-ups between insulin products can occur. Instruct patients to check insulin labels before injection (5.4).
•
Hypersensitivity reactions: Severe, life-threatening, generalized allergy, including anaphylaxis, can occur. Discontinue LEVEMIR, monitor and treat if indicated (5.5).
•
Hypokalemia: May be life-threatening. Monitor potassium levels in patients at risk for hypokalemia and treat if indicated (5.6).
•
Fluid retention and heart failure with concomitant use of thiazolidinediones (TZDs): Observe for signs and symptoms of heart failure; consider dosage reduction or discontinuation if heart failure occurs (5.7).
ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
The following adverse reactions are discussed elsewhere:
•
Hypoglycemia [see Warnings and Precautions (5.3)]
•
Hypoglycemia Due to Medication errors [see Warnings and Precautions (5.4)]
•
Hypersensitivity Reactions [see Warnings and Precautions (5.5)]
•
Hypokalemia [see Warnings and Precautions (5.6)]
6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying designs, the adverse reaction rates reported in one clinical trial may not be easily compared to those rates reported in another clinical trial, and may not reflect the rates actually observed in clinical practice.
The frequencies of adverse reactions (excluding hypoglycemia) reported during LEVEMIR clinical trials in patients with type 1 diabetes mellitus and type 2 diabetes mellitus are listed in Tables 1-4 below. See Tables 5 and 6 for the hypoglycemia findings.
In two pooled trials, adults with type 1 diabetes were exposed to individualized doses of LEVEMIR (n=767) or NPH (n=388). The mean duration of exposure to LEVEMIR was 153 days, and the total exposure to LEVEMIR was 321 patient-years. The most common adverse reactions are summarized in Table 1.
Table 1: Adverse Reactions Occurring in ≥ 5% of LEVEMIR-Treated Adult Patients with Type 1 Diabetes Mellitus in Two Trials of 16 Weeks and 24 Weeks Duration|
LEVEMIR, % (n = 767) | |
|
Upper respiratory tract infection |
26.1 |
|
Headache |
22.6 |
|
Pharyngitis |
9.5 |
|
Influenza-like illness |
7.8 |
|
Abdominal Pain |
6.0 |
Adults with type 1 diabetes were exposed to LEVEMIR (n=161) or insulin glargine (n=159). The mean duration of exposure to LEVEMIR was 176 days, and the total exposure to LEVEMIR was 78 patient-years. The most common adverse reactions are summarized in Table 2.
Table 2: Adverse Reactions Occurring in ≥ 5% of LEVEMIR-Treated Adult Patients with Type 1 Diabetes Mellitus in a 26-week Trial|
LEVEMIR, % (n = 161) | |
|
Upper respiratory tract infection |
26.7 |
|
Headache |
14.3 |
|
Back pain |
8.1 |
|
Influenza-like illness |
6.2 |
|
Gastroenteritis |
5.6 |
|
Bronchitis |
5.0 |
In two pooled trials, adults with type 2 diabetes were exposed to LEVEMIR (n=432) or NPH (n=437). The mean duration of exposure to LEVEMIR was 157 days, and the total exposure to LEVEMIR was 186 patient-years. The most common adverse reactions were comparable to that observed in adult patients with type 1 diabetes mellitus; see Table 1.
Pediatric patients with type 1 diabetes were exposed to individualized doses of LEVEMIR (n=232) or NPH (n=115). The mean duration of exposure to LEVEMIR was 180 days, and the total exposure to LEVEMIR was 114 patient-years. The most common adverse reactions are summarized in Table 3.
Table 3: Adverse Reactions Occurring in ≥ 5% of LEVEMIR-Treated Pediatric Patients with Type 1 Diabetes Mellitus in a 26-week Trial|
LEVEMIR, % (n = 232) | |
|
Upper respiratory tract infection |
35.8 |
|
Headache |
31.0 |
|
Pharyngitis |
17.2 |
|
Gastroenteritis |
16.8 |
|
Influenza-like illness |
13.8 |
|
Abdominal pain |
13.4 |
|
Pyrexia |
10.3 |
|
Cough |
8.2 |
|
Viral infection |
7.3 |
|
Nausea |
6.5 |
|
Rhinitis |
6.5 |
|
Vomiting |
6.5 |
Hypoglycemia
Hypoglycemia was the most commonly observed adverse reaction in patients treated with LEVEMIR. The rates of reported hypoglycemia depend on the definition of hypoglycemia used, diabetes type, insulin dose, intensity of glucose control, background therapies, and other intrinsic and extrinsic patient factors. For these reasons, comparing rates of hypoglycemia in clinical trials for LEVEMIR with the incidence of hypoglycemia for other products may be misleading and also, may not be representative of hypoglycemia rates that will occur in clinical practice.
Table 4 (type 1 diabetes) and Table 5 (type 2 diabetes) summarize the incidence of severe and non-severe hypoglycemia in the LEVEMIR clinical trials.
For the adult trials and one of the pediatric trials (Study D), severe hypoglycemia was defined as an event with symptoms consistent with hypoglycemia requiring assistance of another person and associated with either a plasma glucose value below 56 mg/dL (blood glucose below 50 mg/dL) or prompt recovery after oral carbohydrate, intravenous glucose or glucagon administration. For the other pediatric trial (Study I), severe hypoglycemia was defined as an event with semi-consciousness, unconsciousness, coma and/or convulsions in a patient who could not assist in the treatment and who may have required glucagon or intravenous glucose.
For the adult trials and pediatric trial (Study D), non-severe hypoglycemia was defined as an asymptomatic or symptomatic plasma glucose <56 mg/dL (or equivalently blood glucose <50 mg/dL as used in Study A and C) that was self- treated by the patient. For pediatric Study I, non-severe hypoglycemia included asymptomatic events with plasma glucose <65 mg/dL as well as symptomatic events that the patient could self-treat or treat by taking oral therapy provided by the caregiver.
Table 4: Hypoglycemia in Patients with Type 1 Diabetes|
Severe Hypoglycemia |
Non-Severe Hypoglycemia | ||||
|
Percent of patients with at least 1 event (n/total N) |
Event/patient/ year |
Percent of patients (n/total N) |
Event/patient/ year | ||
|
Study A Type 1 Diabetes Adults 16 weeks In combination with insulin aspart |
Twice-Daily LEVEMIR |
8.7 (24/276) |
0.52 |
88.0 (243/276) |
26.4 |
|
Study B Type 1 Diabetes Adults 26 weeks In combination with insulin aspart |
Twice-Daily LEVEMIR |
5.0 (8/161) |
0.13 |
82.0 (132/161) |
20.2 |
|
Study C Type 1 Diabetes Adults 24 weeks In combination with regular insulin |
Once-Daily LEVEMIR |
7.5 (37/491) |
0.35 |
88.4 (434/491) |
31.1 |
|
Study D Type 1 Diabetes Pediatrics 26 weeks In combination with insulin aspart |
Once- or Twice Daily LEVEMIR |
15.9 (37/232) |
0.91 |
93.1 (216/232) |
31.6 |
|
Study I Type 1 Diabetes Pediatrics 52 weeks In combination with insulin aspart |
Once- or Twice Daily LEVEMIR |
1.7 (3/177) |
0.02 |
94.9 (168/177) |
56.1 |
|
Study E Type 2 Diabetes Adults 24 weeks In combination with oral agents |
Study F Type 2 Diabetes Adults 22 weeks In combination with insulin aspart |
Study H Type 2 Diabetes Adults 26 weeks in combination with Liraglutide and Metformin | ||
|
Twice-Daily LEVEMIR |
Once- or Twice Daily LEVEMIR |
Once Daily LEVEMIR + Liraglutide + Metformin | ||
|
Severe hypoglycemia |
Percent of patients with at least 1 event (n/total N) |
0.4 (1/237) |
1.5 (3/195) |
0 |
|
Event/patient/year |
0.01 |
0.04 |
0 | |
|
Non-severe hypoglycemia |
Percent of patients (n/total N) |
40.5 (96/237) |
32.3 (63/195) |
9.2 (15/163) |
|
Event/patient/year |
3.5 |
1.6 |
0.29 |
Hypersensitivity Reactions
Severe, life-threatening, generalized allergy, including anaphylaxis, generalized skin reactions, angioedema, bronchospasm, hypotension, and shock have occurred with insulin, including LEVEMIR, and may be life-threatening.
Insulin Initiation and Intensification of Glucose Control
Intensification or rapid improvement in glucose control has been associated with a transitory, reversible ophthalmologic refraction disorder, worsening of diabetic retinopathy, and acute painful peripheral neuropathy. However, long- term glycemic control decreases the risk of diabetic retinopathy and neuropathy.
Lipodystrophy
Long-term use of insulin, including LEVEMIR, can cause lipodystrophy at the site of repeated insulin injections. Lipodystrophy includes lipohypertrophy (thickening of adipose tissue) and lipoatrophy (thinning of adipose tissue), and may affect insulin absorption [see Dosage and Administration (2.1)].
Weight Gain
Weight gain can occur with insulin therapy, including LEVEMIR, and has been attributed to the anabolic effects of insulin and the decrease in glucosuria [see Clinical Studies (14)]. In the clinical program, the mean change in body weight from baseline in adult patients with type 1 diabetes (Study A, B, and C) treated with LEVEMIR ranged from -0.3 kg to 0.5 kg. The mean change in body weight from baseline in adult patients with type 2 diabetes (Study E, F, and H) treated with LEVEMIR ranged from 0.5 kg to 1.2 kg.
Peripheral Edema
Insulin, including LEVEMIR, may cause sodium retention and edema, particularly if previously poor metabolic control is improved by intensified insulin therapy.
Injection Site Reactions
Patients taking LEVEMIR may experience injection site reactions, including localized erythema, pain, pruritus, urticaria, edema, and inflammation. In clinical studies in adults, three patients treated with LEVEMIR reported injection site pain (0.25%).
Immunogenicity
All insulin products can elicit the formation of insulin antibodies. These insulin antibodies may increase or decrease the efficacy of insulin and may require adjustment of the insulin dose. In clinical trials of LEVEMIR, antibody development has been observed with no apparent impact on glycemic control.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use of LEVEMIR. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Medication errors have been reported in which rapid-acting or short-acting insulins and other insulins, have been accidentally administered instead of LEVEMIR.
Localized cutaneous amyloidosis at the injection site has occurred. Hyperglycemia has been reported with repeated insulin injections into areas of localized cutaneous amyloidosis; hypoglycemia has been reported with a sudden change to an unaffected injection site.
Adverse reactions associated with LEVEMIR include hypoglycemia, allergic reactions, injection site reactions, lipodystrophy, rash and pruritus (6).
To report SUSPECTED ADVERSE REACTIONS, contact Novo Nordisk Inc. at 1-800-727-6500 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
Table 6 includes clinically significant drug interactions with LEVEMIR.
Table 6: Clinically Significant Drug Interactions with LEVEMIR
|
Drugs That May Increase the Risk of Hypoglycemia | |
|
Drugs: |
Antidiabetic agents, ACE inhibitors, angiotensin II receptor blocking agents, disopyramide, fibrates, fluoxetine, monoamine oxidase inhibitors, pentoxifylline, pramlintide, salicylates, somatostatin analogs (e.g., octreotide), and sulfonamide antibiotics, GLP-1 receptor agonists, DPP-4 inhibitors, SGLT-2 inhibitors. |
|
Intervention: |
Dosage reductions and increased frequency of glucose monitoring may be required when LEVEMIR is co-administered with these drugs. |
|
** Drugs That May Decrease the Blood Glucose Lowering Effect of LEVEMIR** | |
|
Drugs: |
Atypical antipsychotics (e.g., olanzapine and clozapine), corticosteroids, danazol, diuretics, estrogens, glucagon, isoniazid, niacin, oral contraceptives, phenothiazines, progestogens (e.g., in oral contraceptives), protease inhibitors, somatropin, sympathomimetic agents (e.g., albuterol, epinephrine, terbutaline), and thyroid hormones. |
|
Intervention: |
Dosage increases and increased frequency of glucose monitoring may be required when LEVEMIR is co-administered with these drugs. |
|
** Drugs That May Increase or Decrease the Blood Glucose Lowering Effect of LEVEMIR** | |
|
Drugs: |
Alcohol, beta-blockers, clonidine, and lithium salts. Pentamidine may cause hypoglycemia, which may sometimes be followed by hyperglycemia. |
|
Intervention: |
Dosage adjustment and increased frequency of glucose monitoring may be required when LEVEMIR is co-administered with these drugs. |
|
** Drugs That May Blunt Signs and Symptoms of Hypoglycemia** | |
|
Drugs: |
Beta-blockers, clonidine, guanethidine, and reserpine |
|
Intervention: |
Increased frequency of glucose monitoring may be required when LEVEMIR is co- administered with these drugs. |
•
Drugs that Affect Glucose Metabolism: Adjustment of insulin dosage may be needed. (7)
•
Antiadrenergic Drugs (e.g., beta-blockers, clonidine, guanethidine, and reserpine): Signs and symptoms of hypoglycemia may be reduced or absent. (5.3, 7)
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Standard 2-year carcinogenicity studies in animals have not been performed. Insulin detemir tested negative for genotoxic potential in the in vitro reverse mutation study in bacteria, human peripheral blood lymphocyte chromosome aberration test, and the in vivo mouse micronucleus test.
In a fertility and embryonic development study, insulin detemir was administered to female rats before mating, during mating, and throughout pregnancy at doses up to 300 nmol/kg/day (3 times a human dose of 0.5 units/kg/day, based on plasma AUC ratio). There were no effects on fertility in the rat.
SPL PATIENT PACKAGE INSERT SECTION
Patient Information
|
PATIENT INFORMATION LEVEMIR**®**** (LEV–uh-mere)** (insulin detemir) injection, for subcutaneous use |
|
Do not share your LEVEMIR FlexPen with other people, even if the needle has been changed. Do not share needles or syringes with another person. You may give other people a serious infection or get a serious infection from them. |
|
What is LEVEMIR? • • |
|
Who should not take LEVEMIR? Do not take LEVEMIR if you: • • |
|
Before taking LEVEMIR, tell your healthcare provider about all your medical conditions including, if you: • • **Tell your healthcare provider all the medicines you take,**including prescription and over-the-counter medicines, vitamins, or herbal supplements. Before you start taking LEVEMIR, talk to your healthcare provider about low blood sugar and how to manage it. |
|
How should I take LEVEMIR? • • • • • • • • o o o |
|
What should I avoid while taking LEVEMIR? While taking LEVEMIR do not: • • |
|
What are the possible side effects of LEVEMIR? LEVEMIR may cause serious side effects that can lead to death, including: • • • • • • • • • • • • • • • • • • • • • • • • • • Treatment with TZDs and LEVEMIR may need to be changed or stopped by your healthcare provider if you have new or worse heart failure. Other common side effects of LEVEMIR include: • • • • • • Your insulin dose may need to change because of: • • • • • Tell your healthcare provider if you have any side effect that bothers you or that does not go away. These are not all the possible side effects of LEVEMIR. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
|
General information about the safe and effective use of LEVEMIR. Medicines are sometimes prescribed for purposes other than those listed in a Patient Information Leaflet. Do not use LEVEMIR for a condition for which it was not prescribed. Do not give LEVEMIR to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about LEVEMIR that is written for health professionals. |
|
What are the ingredients in LEVEMIR? **Active Ingredient:**insulin detemir **Inactive Ingredients:**dibasic sodium phosphate, glycerin, metacresol, phenol, sodium chloride, zinc and Water for Injection, USP. Hydrochloric acid or sodium hydroxide may be added. Manufactured by: Novo Nordisk Inc. 800 Scudders Mill Road Plainsboro, NJ 08536 U.S. License Number 1261 For more information, go to www.novonordisk-us.com or call 1-800-727-6500. |
This Patient Information has been approved by the U.S. Food and Drug Administration.
Revised: 12/2022
INSTRUCTIONS FOR USE
LEVEMIR®** (LEV-uh-mere)**
injection, for subcutaneous use
10 mL multiple-dose vial
Please read the following Instructions for use carefully before using your LEVEMIR® vial and each time you get a refill. You should read the instructions in this manual even if you have used an insulin vial before.
How should I use the LEVEMIR vial?
Using the vial:
|
1. Check to make sure that you have the correct type of insulin. This is especially important if you use different types of insulin. | |
|
2. Look at the vial and the insulin. The LEVEMIR insulin should be clear and colorless. The tamper-resistant cap should be in place before the first use. If the cap has been removed before your first use of the vial, or if the insulin is cloudy or colored,do not use the insulin and return it to your pharmacy. | |
|
3. Wash your hands with soap and water. | |
|
4. If you are using a new vial, pull off the tamper-resistant cap. Before each use, wipe the rubber stopper with an alcohol wipe. |
|
|
5. Do not roll or shake the vial. Shaking the vial right before the dose is drawn into the syringe may cause bubbles or foam. This can cause you to draw up the wrong dose of insulin. The insulin should be used only if it is clear and colorless. | |
|
6. Pull back the plunger on your syringe until the black tip reaches the marking for the number of units you will inject. |
|
|
7. Push the needle through the rubber stopper into the vial. |
|
|
8. Push the plunger all the way in. This inserts air into the vial. |
|
|
9. Turn the vial and syringe upside down and slowly pull the plunger back to a few units beyond the correct dose that you need. |
|
|
10. If there are air bubbles, tap the syringe gently with your finger to raise the air bubbles to the top of the needle. Then slowly push the plunger to the correct unit marking for your dose. |
|
|
11. Check to make sure you have the right dose of LEVEMIR in the syringe. | |
|
12. Pull the syringe out of the vial. | |
|
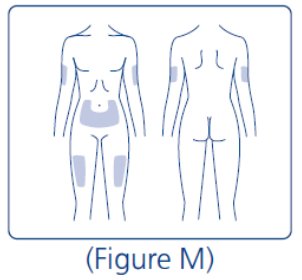
13. Inject your LEVEMIR right away as instructed by your healthcare provider. LEVEMIR can be injected under the skin (subcutaneously) of your stomach area (abdomen), upper legs (thighs) or upper arms. For each injection, change (rotate) your injection site within the area of skin that you use to reduce your risk of getting lipodystrophy (pits in skin or thickened skin) and localized cutaneous amyloidosis (skin with lumps) at the injection sites.Do not use the same injection site for each injection.Do not inject where the skin has pits, is thickened, or has lumps.Do not inject where the skin is tender, bruised, scaly or hard, or into scars or damaged skin. |
How should I inject LEVEMIR with a syringe?
If you clean your injection site with an alcohol swab, let the injection site dry before you inject. Talk with your healthcare provider about how to rotate injection sites and how to give an injection.
|
1. Pinch your skin between two fingers, push the needle into the skinfold, using a dart-like motion and push the plunger to inject the insulin under your skin. The needle will be straight in. |
|
|
2. Keep the needle under your skin for at least 6 seconds to make sure you have injected all the insulin. After you pull the needle from your skin you may see a drop of LEVEMIR at the needle tip. This is normal and has no effect on the dose you just received. | |
|
3. If blood appears after you pull the needle from your skin, press the injection site lightly with an alcohol swab. Do not rub the area. | |
|
4. After each injection,remove the needle without recapping and dispose of it in a puncture-resistant container. Used vials, syringes, needles, and lancets should be placed in sharps containers (such as red biohazard containers), hard plastic containers (such as detergent bottles), or metal containers (such as an empty coffee can). Such containers should be sealed and disposed of properly. How should I store LEVEMIR? • • • o o o • o o |
Revised: 07/2022
Novo Nordisk® and LEVEMIR® are registered trademarks of Novo Nordisk A/S.
PATENT Information: https://www.novonordisk-us.com/products/product- patents.html
© 2005-2022 Novo Nordisk
Manufactured by:
Novo Nordisk Inc.
800 Scudders Mill Road
Plainsboro, NJ 08536
U.S. License Number 1261
INSTRUCTIONS FOR USE
Levemir**®**** (LEV–uh-mere)**
(insulin detemir)
injection, for subcutaneous use
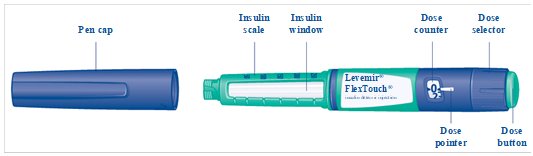
FlexTouch**®**Pen
Please read the following instructions carefully before using your Levemir FlexTouch Pen.
•
**Do not share your Levemir FlexTouch Pen with other people, even if the needle has been changed. You may give other people a serious infection, or get a serious infection from them.**
•
**Levemir FlexTouch Pen (“Pen”) is a prefilled disposable, single-patient-use insulin pen**containing 300 units insulin detemir. You can inject from 1 to 80 units in a single injection.
•
**People who are blind or have vision problems should not use this Pen without help from a person trained to use the Pen.**
Supplies you will need to give your Levemir injection:
•
Levemir FlexTouch Pen
•
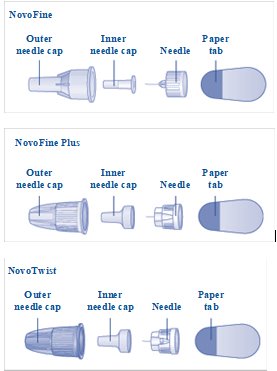
a new NovoFine, NovoFine Plus or NovoTwist needle
•
alcohol swab
•
1 sharps container for throwing away used Pens and needles.**See “Disposing of used Levemir FlexTouch Pens and needles” at the end of these instructions.**
Preparing your Levemir FlexTouch Pen:
•
Wash your hands with soap and water.
•
**Before you start to prepare your injection, check the Levemir FlexTouch Pen label to make sure you are taking the right type of insulin. This is especially important if you take more than 1 type of insulin.**
•
Levemir should look clear and colorless.**Do not** use Levemir if it is thick, cloudy, or is colored.
•
**Do not** use Levemir past the expiration date printed on the label or 42 days after you start using the Pen.
•
**Always**use a new needle for each injection to help ensure sterility and prevent blocked needles.**Do not reuse or share your needles with other people. You may give other people a serious infection, or get a serious infection from them.**
|
Step 1: • |
|
|
Step 2: • |
|
|
Step 3: • • |
|
|
Step 4: • |
|
|
Step 5: • |
|
|
Step 6: • |
|
Priming your Levemir FlexTouch Pen:
|
Step 7: • |
|
|
Step 8: • |
|
|
Step 9: • • o o |
|
Selecting your dose:
|
Step 10: • o o o |
|
|
• • o o |
|
Giving your injection:
•
Inject your Levemir exactly as your healthcare provider has shown you. Your healthcare provider should tell you if you need to pinch the skin before injecting.
•
Levemir can be injected under the skin (subcutaneously) of your stomach area (abdomen), upper legs (thighs) or upper arms.
•
For each injection, change (rotate) your injection site within the area of skin that you use to reduce your risk of getting lipodystrophy (pits in skin or thickened skin) and localized cutaneous amyloidosis (skin with lumps) at the injection sites.**Do not** use the same injection site for each injection.**Do not** inject where the skin has pits, is thickened, or has lumps.**Do not** inject where the skin is tender, bruised, scaly or hard, or into scars or damaged skin.
|
Step 11: • |
|
|
Step 12: • o |
|
|
Step 13: • o |
|
|
• o o o |
|
|
Step 14: • o |
|
|
Step 15: • o • o |
|
|
Step 16: • |
|
After your injection:
•
The used Levemir FlexTouch Pen may be thrown away in your household trash after you have removed the needle.
•
You can put your used needles in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) loose needles in your household trash.
•
If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
o
made of a heavy-duty plastic
o
can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out
o
upright and stable during use
o
leak-resistant
o
properly labeled to warn of hazardous waste inside the container
•
When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. Do not reuse or share your needles or syringes with other people. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at: <http://www.fda.gov/safesharpsdisposal>.
•
Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
How should I store my Levemir FlexTouch Pen?
•
Store unused Levemir FlexTouch Pens in the refrigerator at 36°F to 46°F (2°C to 8°C).
•
Store the Pen you are currently using out of the refrigerator up to 86°F.
•
**Do not** freeze Levemir.**Do not** use Levemir if it has been frozen.
•
Keep Levemir away from heat or light.
•
Unused Pens may be used until the expiration date printed on the label, if kept in the refrigerator.
•
The Levemir FlexTouch Pen you are using should be thrown away after 42 days, even if it still has insulin left in it.
General Information about the safe and effective use of Levemir:
•
**Keep Levemir FlexTouch Pens and needles out of the reach of children.**
•
**Always** use a new needle for each injection.
•
**Do not** share your Levemir FlexTouch Pen or needles with other people. You may give other people a serious infection, or get a serious infection from them.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured by:
Novo Nordisk Inc.
800 Scudders Mill Road
Plainsboro, NJ 08536
U.S. License Number 1261
Revised: 07/2022

For more information go to
www.novotraining.com/levemirflextouch/us02
© 2005-2022 Novo Nordisk
Levemir**®**
FlexTouch®
Read before first use
Instructions For Use
LEVEMIR**®**** (LEV–uh-mere) FlexPen****®**
(insulin detemir)
injection, for subcutaneous use
Please read the following instructions carefully before using your LEVEMIR FlexPen.
Do not share your LEVEMIR FlexPen with other people, even if the needle has been changed. You may give other people a serious infection or get a serious infection from them.
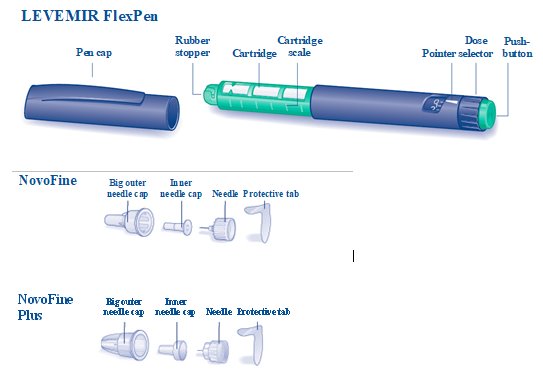
LEVEMIR FlexPen is a prefilled disposable, single-patient-use, insulin pen. You can select doses from 1 to 60 units in increments of 1 unit. LEVEMIR FlexPen is designed to be used with NovoFine or NovoFine Plus needles.
 People
who are blind or have vision problems should not use this Pen without help
from a person trained to use the Pen.
People
who are blind or have vision problems should not use this Pen without help
from a person trained to use the Pen.
Getting ready
Make sure you have the following items:
▪
LEVEMIR FlexPen
▪
NovoFine or NovoFine Plus disposable needles
▪
Alcohol swab
▪
Sharps disposal container (see**After the Injection**)
Preparing your LEVEMIR FlexPen
Wash your hands with soap and water. Before you start to prepare your injection, check the label to make sure that you are taking the right type of insulin. This is especially important if you take more than 1 type of insulin. LEVEMIR should look clear and colorless.Do not use LEVEMIR if it is thick, cloudy, or is colored.
|
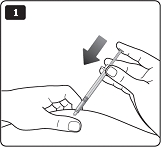
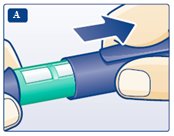
**A.**Pull off the pen cap (see diagram A). Wipe the rubber stopper with an alcohol swab. |
|
|
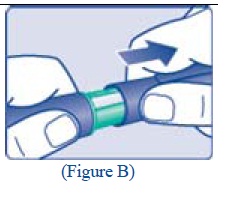
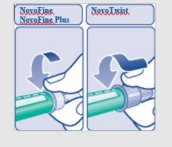
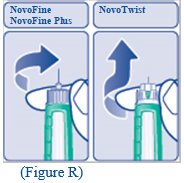
B. Attaching the needle Remove the protective tab from a new disposable needle. |
|
Screw the needle tightly onto your FlexPen. It is important that the needle is put on straight (see diagram B). Never place a disposable needle on your LEVEMIR FlexPen until you are ready to give your injection. |
|
|
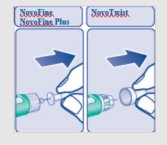
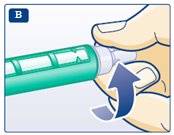
**C.**Pull off the big outer needle cap (see diagram C). |
|
|
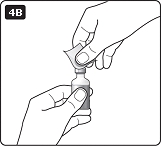
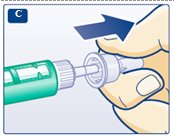
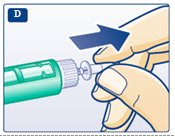
**D.**Pull off the inner needle cap and throw it away (dispose of it) (see diagram D). |
|
 Always use a new
needle for each injection to make sure the needle is free of germs (sterile)
and to prevent blocked needles. Do not reuse or share your needles with other
people. You may give other people a serious infection or get a serious
infection from them.
Always use a new
needle for each injection to make sure the needle is free of germs (sterile)
and to prevent blocked needles. Do not reuse or share your needles with other
people. You may give other people a serious infection or get a serious
infection from them.
 Be careful not
to bend or damage the needle before use.
Be careful not
to bend or damage the needle before use.
 To
reduce the risk of needle sticks,** never put the inner needle cap back on the
needle.**
To
reduce the risk of needle sticks,** never put the inner needle cap back on the
needle.**
Giving the airshot before each injection
Before each injection, small amounts of air may collect in the cartridge during normal use. To avoid injecting air and to ensure proper dosing:
|
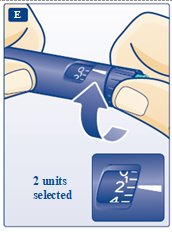
**E.**Turn the dose selector to select 2 units (see diagram E). |
|
|
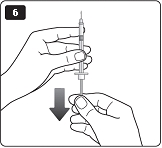
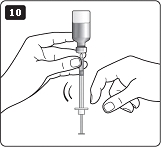
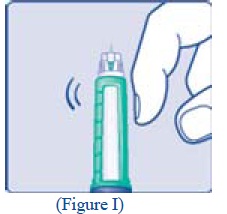
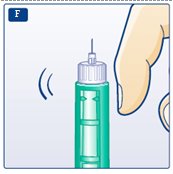
**F.**Hold your LEVEMIR FlexPen with the needle pointing up. Tap the cartridge gently with your finger a few times to make any air bubbles collect at the top of the cartridge (see diagram F). |
|
|
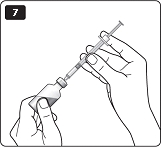
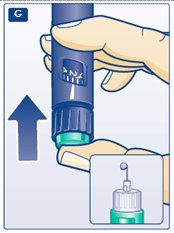
**G.**Keep the needle pointing upwards, press the green push-button all the way in (see diagram G). The dose selector returns to 0. A drop of insulin should appear at the needle tip. If not, change the needle and repeat the procedure no more than 6 times. If you do not see a drop of insulin after 6 times, do not use the LEVEMIR FlexPen and contact Novo Nordisk at 1-800-727-6500. A small air bubble may remain at the needle tip, but it will not be injected. |
|
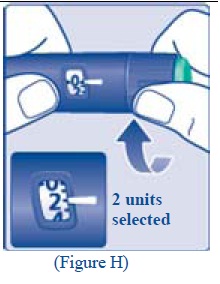
Selecting your dose
Check and make sure that the dose selector is set at 0.
|
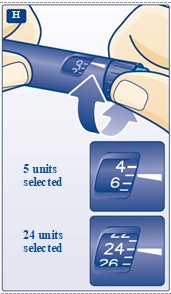
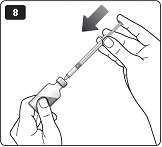
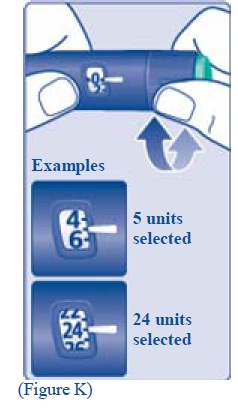
**H.**Turn the dose selector to the number of units you need to inject. The pointer should line up with your dose. The dose can be corrected either up or down by turning the dose selector in either direction until the correct dose lines up with the pointer (see diagram H). When turning the dose selector, be careful not to press the green push- button as insulin will come out. You cannot select a dose larger than the number of units left in the cartridge. You will hear a click for every single unit dialed.Do not set the dose by counting the number of clicks you hear because you may get an incorrect dose.
|
|
Giving the injection
Give the injection exactly as shown to you by your healthcare provider. Your healthcare provider should tell you if you need to pinch the skin before injecting. Wipe the skin with an alcohol swab and let the area dry.
LEVEMIR can be injected under the skin (subcutaneously) of your stomach area (abdomen), upper legs (thighs), or upper arms.
Change (rotate) your injection sites within the area you choose for each dose to reduce your risk of getting lipodystrophy (pits in skin or thickened skin) and localized cutaneous amyloidosis (skin with lumps) at the injection sites. Do not use the same injection site for each injection.Do not inject where the skin has pits, is thickened, or has lumps.Do not inject where the skin is tender, bruised, scaly or hard, or into scars or damaged skin.
|
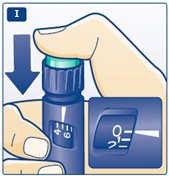
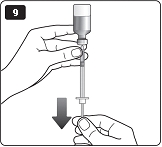
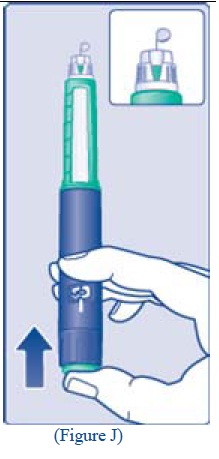
** I.**Insert the needle into your skin. Inject the dose by pressing the green push-button all the way in until the 0 lines up with the pointer (see diagram I). Be careful only to push the green push-button when injecting. Turning the dose selector will not inject the insulin. |
|
|
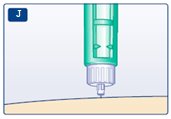
**J.**Keep the needle in the skin for at least 6 seconds, and keep the green push-button pressed all the way in until the needle has been pulled out from the skin (see diagram J). This will make sure that the full dose has been given. You may see a drop of insulin at the needle tip. This is normal and has no effect on the dose you just received. If blood appears after you take the needle out of your skin, press the injection site lightly with an alcohol swab.** Do not rub the area.** |
|
After the injection
**Do not recap the needle.**Recapping can lead to a needle stick injury. Remove the needle from the LEVEMIR FlexPen after each injection and dispose of it. This helps to prevent infection, leakage of insulin, and will help to make sure you inject the right dose of insulin.
•
If you do not have a sharps container, carefully slip the needle into the outer needle cap using 1 hand. Use your other hand to pinch the base of the big outer needle cap and unscrew the used needle from the Pen and throw it away as soon as you can.
•
The used LEVEMIR FlexPen may be thrown away in your household trash after you have removed the needle.
•
Put your used needles in an FDA-cleared sharps disposal container right away after use.**Do not** throw away (dispose of) loose needles in your household trash.
•
If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
o
made of a heavy-duty plastic,
o
can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
o
upright and stable during use,
o
leak-resistant, and
o
properly labeled to warn of hazardous waste inside the container.
•
When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about the safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at: <http://www.fda.gov/safesharpsdisposal>.
Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this.Do not recycle your used sharps disposal container.
When there is not enough medicine left in your LEVEMIR FlexPen for your prescribed dose, the LEVEMIR FlexPen may be thrown away in your household trash after you have removed the needle.
The LEVEMIR FlexPen prevents the cartridge from being completely emptied. It is designed to deliver 300 units.
|
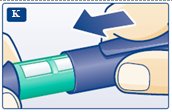
**K.**Put the pen cap on the LEVEMIR FlexPen and store the LEVEMIR FlexPen without the needle attached (see diagram K). Storing without the needle attached helps prevent leaking, blocking of the needle, and air from entering the LEVEMIR FlexPen. |
|
How should I store LEVEMIR FlexPen?
•
Store the unused (unopened) LEVEMIR FlexPen in the refrigerator between 36°F to 46°F (2°C to 8°C).
•
Store the LEVEMIR FlexPen you are currently using out of the refrigerator up to 86°F.
•
**Do not** freeze the LEVEMIR FlexPen.**Do not** use the LEVEMIR FlexPen if it has been frozen.
•
Keep the LEVEMIR FlexPen away from heat or light.
•
Unused LEVEMIR FlexPens may be used until the expiration date printed on the label, if kept in the refrigerator.
The LEVEMIR FlexPen you are using should be thrown away after 42 days, even if it still has insulin left in it.
Keep your LEVEMIR FlexPen and needles out of the reach of children.
Maintenance
For the safe and proper use of your LEVEMIR FlexPen be sure to handle it with care. Avoid dropping your LEVEMIR FlexPen as it may damage it. If you are concerned that your LEVEMIR FlexPen is damaged, use a new one. You can clean the outside of your LEVEMIR FlexPen by wiping it with a damp cloth.Do not soak or wash your LEVEMIR FlexPen as it may damage it.Do not refill your LEVEMIR FlexPen.
 Remove
the needle from the LEVEMIR FlexPen after each injection. This helps to ensure
sterility, prevent leakage of insulin, and will help to make sure you inject
the right dose of insulin for future injections.
Remove
the needle from the LEVEMIR FlexPen after each injection. This helps to ensure
sterility, prevent leakage of insulin, and will help to make sure you inject
the right dose of insulin for future injections.
 Be
careful when handling used needles to avoid needle sticks and transfer of
infectious diseases.
Be
careful when handling used needles to avoid needle sticks and transfer of
infectious diseases.
 Use the
LEVEMIR FlexPen exactly as your healthcare provider tells you to.
Use the
LEVEMIR FlexPen exactly as your healthcare provider tells you to.
 ** Do
not** share your LEVEMIR FlexPen or needles with other people. You may give
other people a serious infection, or get a serious infection from them.
** Do
not** share your LEVEMIR FlexPen or needles with other people. You may give
other people a serious infection, or get a serious infection from them.
 Always
use a new needle for each injection.
Always
use a new needle for each injection.
 Novo
Nordisk is not responsible for harm due to using this insulin pen with
products not recommended by Novo Nordisk.
Novo
Nordisk is not responsible for harm due to using this insulin pen with
products not recommended by Novo Nordisk.
 As a
precautionary measure, always carry a spare insulin delivery device in case
your LEVEMIR FlexPen is lost or damaged.
As a
precautionary measure, always carry a spare insulin delivery device in case
your LEVEMIR FlexPen is lost or damaged.
 Remember
to keep the disposable LEVEMIR FlexPen with you.Do not leave it in a car
or other location where it can get too hot or too cold.
Remember
to keep the disposable LEVEMIR FlexPen with you.Do not leave it in a car
or other location where it can get too hot or too cold.
© 2022 Novo Nordisk
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured by:
Novo Nordisk Inc.
800 Scudders Mill Road
Plainsboro, NJ 08536
U.S. License Number 1261
Revised: 12/2022



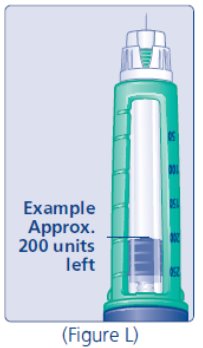
 Do not use
the cartridge scale printed on the cartridge to measure your dose of insulin.
Do not use
the cartridge scale printed on the cartridge to measure your dose of insulin.