Hydrochlorothiazide
Hydrochlorothiazide Tablets, USP
d7d0867a-748e-4960-851f-ad764e07f774
HUMAN PRESCRIPTION DRUG LABEL
Aug 20, 2025
Bryant Ranch Prepack
DUNS: 171714327
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Hydrochlorothiazide
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
Drug Labeling Information
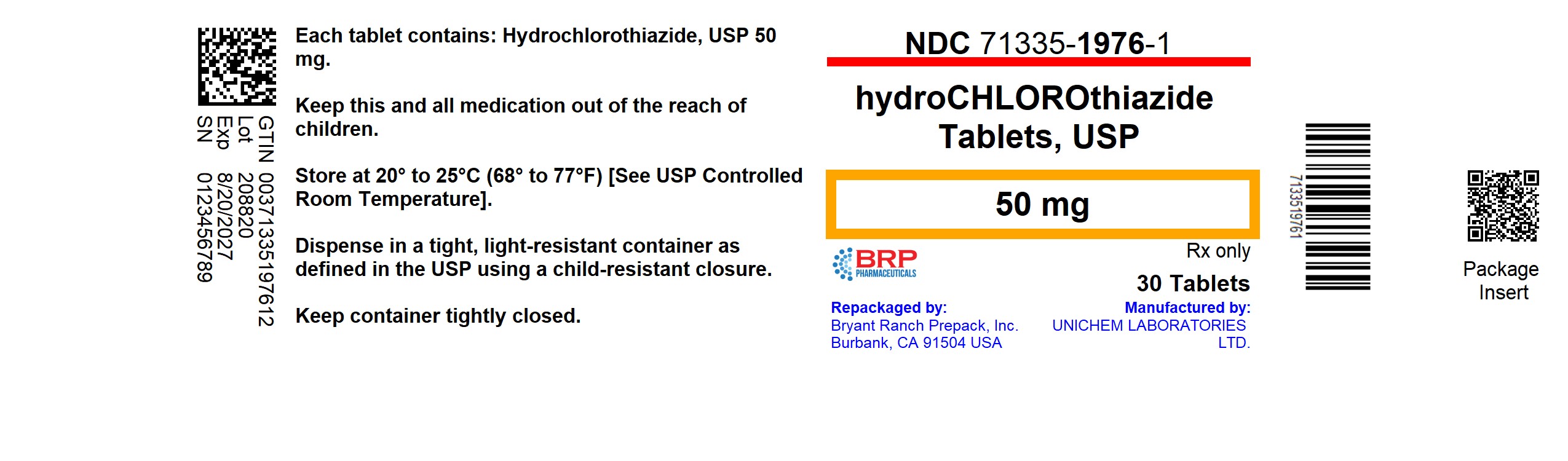
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Hydrochlorothiazide 50mg Tablet
DESCRIPTION SECTION
DESCRIPTION
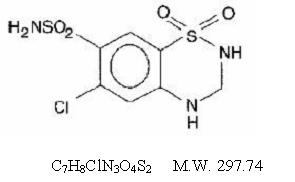
Hydrochlorothiazide, USP is a diuretic and antihypertensive. It is the 3,4-dihydro derivative of chlorothiazide. It is chemically designated as 6-chloro-3,4-dihydro-2H-1,2,4- benzothiadiazine-7-sulfonamide 1,1-dioxide and has the following structural formula:
Hydrochlorothiazide, USP is a white, or practically white, crystalline powder which is slightly soluble in water, freely soluble in sodium hydroxide solution, in n-butylamine, and in dimethylformamide; sparingly soluble in methanol; insoluble in ether, in chloroform, and in dilute mineral acids. Each tablet for oral administration contains 25 mg or 50 mg hydrochlorothiazide, USP. In addition, each tablet contains the following inactive ingredients: corn starch, FD&C Yellow #6, dibasic calcium phosphate, pregelatinized starch, colloidal silicon dioxide, lactose monohydrate and magnesium stearate.
HOW SUPPLIED SECTION
HOW SUPPLIED
Hydrochlorothiazide Tablets, USP are available as light orange, circular, flat, beveled, uncoated tablets, with score line having "U" and "129" debossed across the score line on one side and plain on other side containing 50 mg of hydrochlorothiazide, USP.
NDC: 71335-1976-1: 30 Tablets in a BOTTLE
NDC: 71335-1976-2: 60 Tablets in a BOTTLE
NDC: 71335-1976-3: 90 Tablets in a BOTTLE
NDC: 71335-1976-4: 28 Tablets in a BOTTLE
NDC: 71335-1976-5: 56 Tablets in a BOTTLE
NDC: 71335-1976-6: 100 Tablets in a BOTTLE
NDC: 71335-1976-7: 7 Tablets in a BOTTLE
NDC: 71335-1976-8: 14 Tablets in a BOTTLE
NDC: 71335-1976-9: 120 Tablets in a BOTTLE
NDC: 71335-1976-0: 45 Tablets in a BOTTLE
PHARMACIST: Dispense in a well-closed container as defined in the USP. Use child-resistant closure (as required)
Store at 20° to 25° C (68° to 77° F)
[see USP Controlled Room Temperature].
KEEP THIS AND ALL DRUGS OUT OF REACH OF CHILDREN.
Repackaged/Relabeled by:
Bryant Ranch Prepack, Inc.
Burbank, CA 91504
