COSOPT
These highlights do not include all the information needed to use COSOPT safely and effectively. See full prescribing information for COSOPT. COSOPT (dorzolamide hydrochloride and timolol maleate ophthalmic solution), for topical ophthalmic use Initial U.S. Approval: 1998
23bdf1c1-3134-4ad1-8e7c-bc655156bdc6
HUMAN PRESCRIPTION DRUG LABEL
Sep 5, 2023
Thea Pharma Inc.
DUNS: 117787029
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
dorzolamide hydrochloride and timolol maleate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (8)
Drug Labeling Information
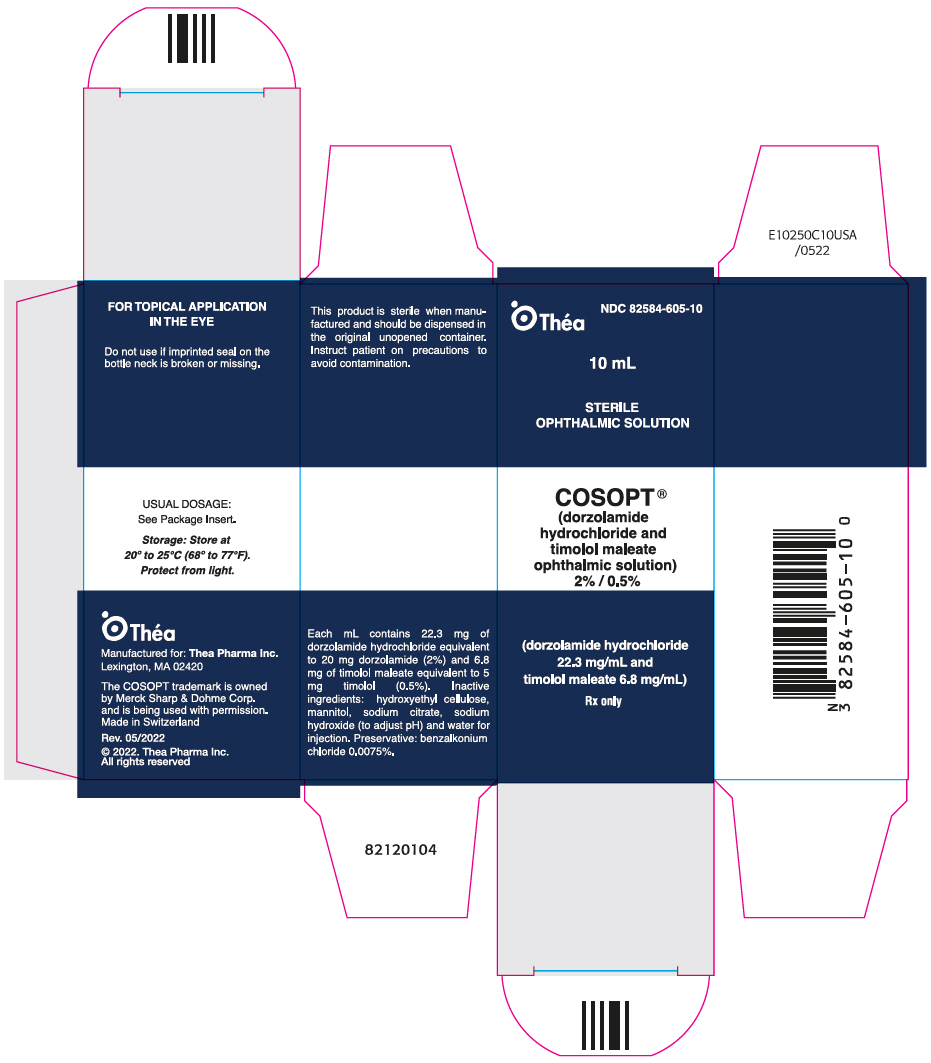
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 10 mL Bottle Carton
NDC 82584-605-10
Théa
10 mL
STERILE
OPHTHALMIC SOLUTION
COSOPT ®
(dorzolamide
hydrochloride and
timolol maleate
ophthalmic solution)
2% / 0.5%
(dorzolamide hydrochloride
22.3 mg/mL and
timolol maleate 6.8 mg/mL)
Rx only
CONTRAINDICATIONS SECTION
4 CONTRAINDICATIONS
4.1 Asthma, COPD
COSOPT is contraindicated in patients with bronchial asthma, a history of bronchial asthma, or severe chronic obstructive pulmonary disease [see Warnings and Precautions (5.1)] .
4.2 Sinus Bradycardia, AV Block, Cardiac Failure, Cardiogenic Shock
COSOPT is contraindicated in patients with sinus bradycardia, second or third degree atrioventricular block, overt cardiac failure, and cardiogenic shock [see Warnings and Precautions (5.2)] .
4.3 Hypersensitivity
COSOPT is contraindicated in patients who are hypersensitive to any component of this product [see Warnings and Precautions (5.3)] .
COSOPT is contraindicated in patients with:
- Bronchial asthma or a history of bronchial asthma, severe chronic obstructive pulmonary disease. ( 4.1)
- Sinus bradycardia, second or third degree atrioventricular block, overt cardiac failure, cardiogenic shock. ( 4.2)
- Hypersensitivity to any component of this product. ( 4.3, 5.3)
USE IN SPECIFIC POPULATIONS SECTION
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Teratogenic Effects. Developmental toxicity studies with dorzolamide hydrochloride in rabbits at oral doses of ≥2.5 mg/kg/day (37 times the recommended human ophthalmic dose) revealed malformations of the vertebral bodies. These malformations occurred at doses that caused metabolic acidosis with decreased body weight gain in dams and decreased fetal weights. No treatment-related malformations were seen at 1 mg/kg/day (15 times the recommended human ophthalmic dose).
Teratogenicity studies with timolol in mice, rats, and rabbits at oral doses up to 50 mg/kg/day (7,000 times the systemic exposure following the maximum recommended human ophthalmic dose) demonstrated no evidence of fetal malformations. Although delayed fetal ossification was observed at this dose in rats, there were no adverse effects on postnatal development of offspring. Doses of 1,000 mg/kg/day (142,000 times the systemic exposure following the maximum recommended human ophthalmic dose) were maternotoxic in mice and resulted in an increased number of fetal resorptions. Increased fetal resorptions were also seen in rabbits at doses of 14,000 times the systemic exposure following the maximum recommended human ophthalmic dose, in this case without apparent maternotoxicity.
There are no adequate and well-controlled studies in pregnant women. COSOPT should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
8.3 Nursing Mothers
It is not known whether dorzolamide is excreted in human milk. Timolol maleate has been detected in human milk following oral and ophthalmic drug administration. Because of the potential for serious adverse reactions from COSOPT in nursing infants, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
8.4 Pediatric Use
The safety and effectiveness of dorzolamide hydrochloride ophthalmic solution and timolol maleate ophthalmic solution have been established when administered individually in pediatric patients aged 2 years and older. Use of these drug products in these children is supported by evidence from adequate and well-controlled studies in children and adults. Safety and efficacy in pediatric patients below the age of 2 years have not be established.
8.5 Geriatric Use
No overall differences in safety or effectiveness have been observed between elderly and younger patients.
DESCRIPTION SECTION
11 DESCRIPTION
COSOPT (dorzolamide hydrochloride and timolol maleate ophthalmic solution) is the combination of a topical carbonic anhydrase inhibitor and a topical beta- adrenergic receptor blocking agent.
Dorzolamide hydrochloride is described chemically as: (4 S-trans)-4-(ethylamino)-5,6-dihydro-6-methyl-4 H-thieno[2,3- b]thiopyran-2-sulfonamide 7,7-dioxide monohydrochloride. Dorzolamide hydrochloride is optically active. The specific rotation is:
|
[α] |
25°C |
(C=1, water) = ~-17°. | |
|
405 nm |
Its empirical formula is C 10H 16N 2O 4S 3 ∙ HCl and its structural formula is:
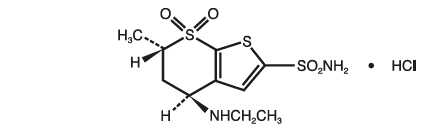
Dorzolamide hydrochloride has a molecular weight of 360.91. It is a white to off-white, crystalline powder, which is soluble in water and slightly soluble in methanol and ethanol.
Timolol maleate is described chemically as: (-)-1-( tert- butylamino)-3-[(4-morpholino-1,2,5-thiadiazol-3-yl)oxy]-2-propanol maleate (1:1) (salt). Timolol maleate possesses an asymmetric carbon atom in its structure and is provided as the levo-isomer. The optical rotation of timolol maleate is:
|
[α] |
25°C |
in 1N HCl (C = 5) = -12.2° (-11.7° to -12.5°). | |
|
405 nm |
Its molecular formula is C 13H 24N 4O 3S ∙ C 4H 4O 4 and its structural formula is:
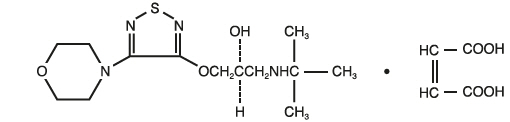
Timolol maleate has a molecular weight of 432.50. It is a white, odorless, crystalline powder which is soluble in water, methanol, and alcohol. Timolol maleate is stable at room temperature.
COSOPT is supplied as a sterile, clear, colorless to nearly colorless, isotonic, buffered, slightly viscous, aqueous solution. The pH of the solution is approximately 5.65, and the osmolarity is 242 to 323 mOsM. Each mL of COSOPT contains 20 mg dorzolamide (equivalent to 22.26 mg of dorzolamide hydrochloride) and 5 mg timolol (equivalent to 6.83 mg timolol maleate). Inactive ingredients are sodium citrate, hydroxyethyl cellulose, sodium hydroxide, mannitol, and water for injection. Benzalkonium chloride 0.0075% is added as a preservative.
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
COSOPT ® (dorzolamide hydrochloride and timolol maleate ophthalmic solution) 2% / 0.5% is supplied in 10 mL white low-density polyethylene (LDPE) plastic bottles with white LDPE dropper tips and blue P/P as follows:
NDC 82584-605-10 10 mL capacity bottle.
Storage: Store at 20° to 25°C (68° to 77°F). Protect from light. After opening, COSOPT can be used until the expiration date on the bottle.
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-Approved patient labeling (Patient Information and Instructions for Use).
Potential for Exacerbation of Asthma and COPD
COSOPT may cause severe worsening of asthma and COPD symptoms including death due to bronchospasm. Advise patients with bronchial asthma, a history of bronchial asthma, or severe chronic obstructive pulmonary disease not to take this product. [see Contraindications (4.1)] .
Potential of Cardiovascular Effects
COSOPT may cause worsening of cardiac symptoms. Advise patients with sinus bradycardia, second or third degree atrioventricular block, or cardiac failure not to take this product. [see Contraindications (4.2)] .
Sulfonamide Reactions
COSOPT contains dorzolamide (which is a sulfonamide) and, although administered topically, is absorbed systemically. Therefore the same types of adverse reactions that are attributable to sulfonamides may occur with topical administration, including severe skin reactions. Advise patients that if serious or unusual reactions or signs of hypersensitivity occur, they should discontinue the use of the product and seek their physician's advise. [see Warnings and Precautions (5.3)] .
Handling Ophthalmic Solutions
Instruct patients that ocular solutions, if handled improperly or if the tip of the dispensing container contacts the eye or surrounding structures, can be contaminated by common bacteria known to cause ocular infections. Serious damage to the eye and subsequent loss of vision may result from using contaminated solutions [see Warnings and Precautions (5.12)] .
Intercurrent Ocular Conditions
Advise patients that if they have ocular surgery or develop an intercurrent ocular condition (e.g., trauma or infection), they should immediately seek their physician's advice concerning the continued use of the present multidose container.
Concomitant Topical Ocular Therapy
If more than one topical ophthalmic drug is being used, the drugs should be administered at least five minutes apart.
Contact Lens Use
Advise patients that COSOPT contains benzalkonium chloride which may be absorbed by soft contact lenses. Contact lenses should be removed prior to administration of the solution. Lenses may be reinserted 15 minutes following administration of COSOPT.
INSTRUCTIONS FOR USE SECTION
INSTRUCTIONS FOR USE
COSOPT ® (CO-sopt)
(dorzolamide hydrochloride and timolol maleate ophthalmic solution)
for topical ophthalmic use
Read this Instructions for Use before you start using COSOPT and each time you get a refill. There may be new information. This leaflet does not take the place of talking to your healthcare provider about your medical condition or treatment.
Important Information:
*COSOPT is for use in the eye.
- If you are using COSOPT with another eyedrop,wait at least 5 minutes before or after using COSOPT.
- If you wear contact lenses, remove them before using COSOPT. The lenses can be placed back into your eyes15 minutes after using COSOPT. *Do not touch your eye or eyelid with the dropper tip. Eye medicines, not handled the right way, can become contaminated by bacteria that can cause eye infections. Serious damage to the eye and followed by loss of vision may happen from using contaminated eye medicines.If you think your COSOPT medicine may be contaminated, or if you develop an eye infection, contact your healthcare provider right away about continuing to use your bottle of COSOPT.
- Wash your hands before each use to make sure you do not infect your eyes while using COSOPT.
- Before using the eyedrops for the first time, be sure the Safety Seal around the cap is not broken. If the Safety Seal is broken, call your pharmacist to get a new bottle of COSOPT.
|
Step 1. |
Tear off the Safety Seal. | ||
|
Step 2. |
To open the COSOPT bottle, unscrew the cap by turning counterclockwise. | ||
|
Step 3. |
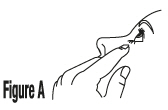
Tilt your head back. Gently pull your lower eyelid downwards to form a pocket between your eyelid and your eye, look up (SeeFigure A). |
| |
|
Step 4. |
Turn the COSOPT bottle upside down. | ||
|
Step 5. |
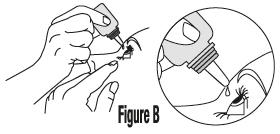
Place the dropper tip of the COSOPT bottle close to your eye but be careful not to touch your eye with it. Gently press the COSOPT bottle lightly with your thumb or index finger until 1 drop of COSOPT falls into your eye (See Figure B). |
| |
|
Step 6. |
RepeatSteps 4 and 5 with the other eye if instructed to do so by your healthcare provider. | ||
|
Step 7. |
Replace the cap by turning until it is firmly touching the bottle.Do not overtighten the cap. | ||
|
Step 8. |
If you use contact lenses,wait at least 15 minutes before placing them back into your eyes. |
- The dropper tip is made to give a single drop of COSOPT.Do not enlarge the hole of the dropper tip.
- After you have used all of your doses of COSOPT, there will be some COSOPT left in the bottle.
- There is an extra amount of COSOPT that has been added to the bottle. You will get the full amount of COSOPT that your doctor prescribed. *Do not try to remove the extra COSOPT medicine from the bottle.
This Instructions for Use has been approved by the U.S. Food and Drug Administration | 11/2020
Manufactured for:Thea Pharma Inc. Lexington, MA 02420
Made in Switzerland
© 2022. Thea Pharma Inc. All rights reserved
The COSOPT trademarks are owned by Merck Sharp & Dohme Corp. and are being
used with permission.
Rev. 05/2022
83120301
N10250C10USA/05/22