Butalbital, Acetaminophen and Caffeine
Butalbital, Acetaminophen and Caffeine Tablets, USP CIII 8467265/0822 Rx only
c2e3eda3-d76f-4332-8260-d2b1939a229a
HUMAN PRESCRIPTION DRUG LABEL
Sep 15, 2025
American Health Packaging
DUNS: 929561009
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Butalbital, Acetaminophen, and Caffeine
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (11)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Package/Label Display Panel – Blister - 50 mg/325 mg/40 mg

Butalbital,CIII
Acetaminophen
and Caffeine Tablet, USP
50 mg/325 mg/40 mg
DESCRIPTION SECTION
DESCRIPTION
Butalbital, Acetaminophen and Caffeine Tablets, USP are supplied in tablet form for oral administration.
Each tablet contains the following active ingredients:
butalbital, USP
......................................................................................
50 mg
acetaminophen, USP
...........................................................................
325 mg
caffeine, USP
........................................................................................
40 mg
Inactive Ingredients: colloidal silicon dioxide, croscarmellose sodium, crospovidone, magnesium stearate, microcrystalline cellulose, povidone, pregelatinized starch, and stearic acid.
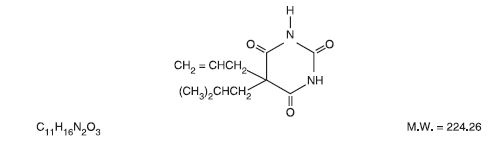
Butalbital (5-allyl-5-isobutylbarbituric acid), is a short to intermediate- acting barbiturate. It has the following structural formula:
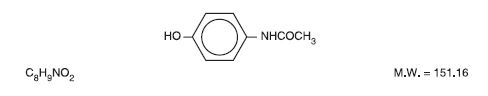
Acetaminophen (4'-hydroxyacetanilide), is a non-opiate, non-salicylate analgesic and antipyretic. It has the following structural formula.
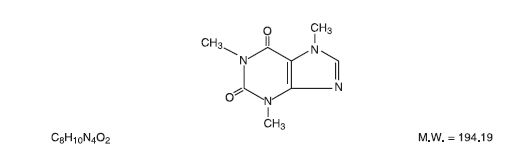
Caffeine (1,3,7-trimethylxanthine), is a central nervous system stimulant. It has the following structural formula: