Esomeprazole Magnesium
ESOMEPRAZOLE delayed-release capsules, for oral use Initial U.S. Approval: 1989 (omeprazole)
71956762-d2f7-4763-9afe-1ca8f32a3cbb
HUMAN PRESCRIPTION DRUG LABEL
Dec 27, 2023
A-S Medication Solutions
DUNS: 830016429
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Esomeprazole Magnesium
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (22)
Drug Labeling Information
WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Presence of Gastric Malignancy
In adults, symptomatic response to therapy with esomeprazole magnesium does not preclude the presence of gastric malignancy. Consider additional follow-up and diagnostic testing in adult patients who have a suboptimal response or an early symptomatic relapse after completing treatment with a PPI. In older patients, also consider an endoscopy.
5.2 Acute Tubulointerstitial Nephritis
Acute tubulointerstitial nephritis (TIN) has been observed in patients taking PPIs and may occur at any point during PPI therapy. Patients may present with varying signs and symptoms from symptomatic hypersensitivity reactions to nonspecific symptoms of decreased renal function (e.g., malaise, nausea, anorexia). In reported case series, some patients were diagnosed on biopsy and in the absence of extra-renal manifestations (e.g., fever, rash or arthralgia). Discontinue esomeprazole magnesium and evaluate patients with suspected acute TIN [see Contraindications (4)].
5.3 Clostridium difficile-Associated Diarrhea
Published observational studies suggest that PPI therapy like esomeprazole magnesium may be associated with an increased risk of Clostridium difficile- associated diarrhea, especially in hospitalized patients. This diagnosis should be considered for diarrhea that does not improve [see Adverse Reactions (6.2)].
Patients should use the lowest dose and shortest duration of PPI therapy appropriate to the condition being treated.
Clostridium difficile-associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents. For more information specific to antibacterial agents (clarithromycin and amoxicillin) indicated for use in combination with esomeprazole magnesium, refer to Warnings and Precautions section of the corresponding prescribing information.
5.4 Bone Fracture
Several published observational studies suggest that proton pump inhibitor (PPI) therapy may be associated with an increased risk for osteoporosis- related fractures of the hip, wrist, or spine. The risk of fracture was increased in patients who received high-dose, defined as multiple daily doses, and long-term PPI therapy (a year or longer). Patients should use the lowest dose and shortest duration of PPI therapy appropriate to the condition being treated. Patients at risk for osteoporosis-related fractures should be managed according to established treatment guidelines [see Dosage and Administration (2) and Adverse Reactions (6.2)].
5.5 Severe Cutaneous Adverse Reactions
Severe cutaneous adverse reactions, including Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN), drug reaction with eosinophilia and systemic symptoms (DRESS), and acute generalized exanthematous pustulosis (AGEP) have been reported in association with the use of PPIs [see Adverse Reactions (6.2)]. Discontinue esomeprazole magnesium at the first signs or symptoms of severe cutaneous adverse reactions or other signs of hypersensitivity and consider further evaluation.
5.6 Cutaneous and Systemic Lupus Erythematosus
Cutaneous lupus erythematosus (CLE) and systemic lupus erythematosus (SLE) have been reported in patients taking PPIs, including esomeprazole. These events have occurred as both new onset and an exacerbation of existing autoimmune disease. The majority of PPI-induced lupus erythematosus cases were CLE.
The most common form of CLE reported in patients treated with PPIs was subacute CLE (SCLE) and occurred within weeks to years after continuous drug therapy in patients ranging from infants to the elderly. Generally, histological findings were observed without organ involvement.
Systemic lupus erythematosus (SLE) is less commonly reported than CLE in patients receiving PPIs. PPI associated SLE is usually milder than non-drug induced SLE. Onset of SLE typically occurred within days to years after initiating treatment primarily in patients ranging from young adults to the elderly. The majority of patients presented with rash; however, arthralgia and cytopenia were also reported.
Avoid administration of PPIs for longer than medically indicated. If signs or symptoms consistent with CLE or SLE are noted in patients receiving esomeprazole magnesium, discontinue the drug and refer the patient to the appropriate specialist for evaluation. Most patients improve with discontinuation of the PPI alone in 4 to 12 weeks. Serological testing (e.g. ANA) may be positive and elevated serological test results may take longer to resolve than clinical manifestations.
5.7 Interaction with Clopidogrel
Avoid concomitant use of esomeprazole magnesium with clopidogrel. Clopidogrel is a prodrug. Inhibition of platelet aggregation by clopidogrel is entirely due to an active metabolite. The metabolism of clopidogrel to its active metabolite can be impaired by use with concomitant medications, such as esomeprazole, that inhibit CYP2C19 activity. Concomitant use of clopidogrel with 40 mg esomeprazole reduces the pharmacological activity of clopidogrel. When using esomeprazole magnesium consider alternative anti-platelet therapy [see Drug Interactions (7)].
5.8 Cyanocobalamin (Vitamin B-12) Deficiency
Daily treatment with any acid-suppressing medications over a long period of time (e.g., longer than 3 years) may lead to malabsorption of cyanocobalamin (vitamin B-12) caused by hypo-or achlorhydria. Rare reports of cyanocobalamin deficiency occurring with acid-suppressing therapy have been reported in the literature. This diagnosis should be considered if clinical symptoms consistent with cyanocobalamin deficiency are observed.
5.9 Hypomagnesemia and Mineral Metabolism
Hypomagnesemia, symptomatic and asymptomatic, has been reported rarely in patients treated with PPIs for at least three months, in most cases after a year of therapy. Serious adverse events include tetany, arrhythmias, and seizures. Hypomagnesemia may lead to hypocalcemia and/or hypokalemia and may exacerbate underlying hypocalcemia in at-risk patients. In most patients, treatment of hypomagnesemia required magnesium replacement and discontinuation of the PPI.
For patients expected to be on prolonged treatment or who take PPIs with medications such as digoxin or drugs that may cause hypomagnesemia (e.g., diuretics), health care professionals may consider monitoring magnesium levels prior to initiation of PPI treatment and periodically [see Adverse Reactions (6.2)]
Consider monitoring magnesium and calcium levels prior to initiation of esomeprazole and magnesium and periodically while on treatment in patients with a preexisting risk of hypocalcemia (e.g., hypoparathyroidism). Supplement with magnesium and/or calcium, as necessary. If hypocalcemia is refractory to treatment, consider discontinuing the PPI.
5.10 Interaction with St. John’s Wort or Rifampin
Drugs which induce CYP2C19 or CYP3A4 (such as St. John’s Wort or rifampin) can substantially decrease esomeprazole concentrations.[see Drug Interactions (7)]. Avoid concomitant use of esomeprazole magnesium with St. John’s Wort, or rifampin.
5.11 Interactions with Diagnostic Investigations for Neuroendocrine Tumors
Serum chromogranin A (CgA) levels increase secondary to drug-induced decreases in gastric acidity. The increased CgA level may cause false positive results in diagnostic investigations for neuroendocrine tumors. Healthcare providers should temporarily stop esomeprazole treatment at least 14 days before assessing CgA levels and consider repeating the test if initial CgA levels are high. If serial tests are performed (e.g. for monitoring), the same commercial laboratory should be used for testing, as reference ranges between tests may vary [see Clinical Pharmacology (12.2)].
5.12 Interaction with Methotrexate
Literature suggests that concomitant use of PPIs with methotrexate (primarily at high dose; see methotrexate prescribing information) may elevate and prolong serum levels of methotrexate and/or its metabolite, possibly leading to methotrexate toxicities. In high-dose methotrexate administration a temporary withdrawal of the PPI may be considered in some patients [see Drug Interactions (7)].
5.13 Fundic Gland Polyps
PPI use is associated with an increased risk of fundic gland polyps that increases with long-term use, especially beyond one year. Most PPI users who developed fundic gland polyps were asymptomatic and fundic gland polyps were identified incidentally on endoscopy. Use the shortest duration of PPI therapy appropriate to the condition being treated.
- Gastric Malignancy:In adults, symptomatic response does not preclude the presence of gastric malignancy. Consider additional follow-up and diagnostic testing. (5.1)
- Acute Tubulointerstitial Nephritis: Discontinue treatment and evaluate patients. (5.2)1
- Clostridium difficile-Associated Diarrhea: PPI therapy may be associated with increased risk (5.3)
- Bone Fracture: Long-term and multiple daily dose PPI therapy may be associated with an increased risk for osteoporosis-related fractures of the hip, wrist or spine. (5.4)
- Severe Cutaneous Adverse Reactions: Discontinue at the first signs or symptoms of severe cutaneous adverse reactions or other signs of hypersensitivity and consider further evaluation. (5.5)
- Cutaneous and Systemic Lupus Erythematosus: Mostly cutaneous; new onset or exacerbation of existing disease; discontinue esomeprazole magnesium and refer to specialist for evaluation. (5.6)
- Interaction with Clopidogrel: Avoid concomitant use of esomeprazole magnesium. (5.7)
- Cyanocobalamin (Vitamin B-12) Deficiency: Daily long-term use (e.g., longer than 3 years) may lead to malabsorption or a deficiency of cyanocobalamin. (5.8)
- Hypomagnesemia and Mineral Metabolism: Reported rarely with prolonged treatment with PPIs. (5.9)
- Interaction with St. John’s Wort or Rifampin: Avoid concomitant use of esomeprazole magnesium. (5.10, 7)
- Interactions with Diagnostic Investigations for Neuroendocrine Tumors: Increased chromogranin A (CgA) levels may interfere with diagnostic investigations for neuroendocrine tumors, temporarily stop esomeprazole magnesium at least 14 days before assessing CgA levels. (5.11, 12.2)
- Interaction with Methotrexate: Concomitant use with PPIs may elevate and/or prolong serum concentrations of methotrexate and/or its metabolite, possibly leading to toxicity. With high dose methotrexate administration, consider temporary withdrawal of esomeprazole magnesium. (5.12, 7)
- Fundic Gland Polyps: Risk increases with long-term use, especially beyond one year. Use the shortest duration of therapy. (5.13)
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
14.1 Healing of EE in Adults
The healing rates of esomeprazole magnesium delayed-release capsules 40 mg, esomeprazole magnesium delayed-release capsules 20 mg, and omeprazole delayed- release capsules 20 mg (the approved dose for this indication) once daily were evaluated in adult patients with endoscopically diagnosed EE in four multicenter, double-blind, randomized studies. The healing rates at Weeks 4 and 8 were evaluated and are shown in Table 11:
Table 11: EE Healing Rate (Life-Table Analysis) in Adults with EE Treated with Esomeprazole Magnesium Delayed-Release Capsules or Omeprazole Delayed- Release Capsules Once Daily in Four Clinical Studies
|
Study |
No. of Patients |
Treatment Groups |
EE Healing Rates |
Significance Level****1 | |
|
Week 4 |
Week 8 | ||||
|
1 |
588 |
Esomeprazole magnesium 20 mg |
68.7% |
90.6% |
N.S. |
|
2 |
654 |
Esomeprazole magnesium 40 mg |
75.9% |
94.1% |
p < 0.001 |
|
3 |
576 |
Esomeprazole magnesium 40 mg |
71.5% |
92.2% |
N.S. |
|
4 |
1216 |
Esomeprazole magnesium 40 mg |
81.7% |
93.7% |
p < 0.001 |
N.S. = not significant (p > 0.05).
1log-rank test vs. omeprazole 20 mg
In these same studies of patients with EE, sustained heartburn resolution and time to sustained heartburn resolution were evaluated and are shown in the Table 12:
Table 12: Sustained Resolution1 of Heartburn in Adults with EE Treated with Esomeprazole Magnesium Delayed-Release Capsules or Omeprazole Delayed-Release Capsules Once Daily in Four Clinical Studies
|
Study |
****No. of Patients |
** Treatment Group** |
Cumulative Percent2 with Sustained Resolution | ||
|
Day 14 |
Day 28 |
Significance Level 3 | |||
|
1 |
573 |
Esomeprazole magnesium 20 mg |
64.3% |
72.7% |
N.S. |
|
2 |
621 |
Esomeprazole magnesium 40 mg |
64.8% |
74.2% |
p <0.001 |
|
3 |
568 |
Esomeprazole magnesium 40 mg |
65.4% |
73.9% |
N.S. |
|
4 |
1187 |
Esomeprazole magnesium 40 mg |
67.6% |
75.1% |
p <0.001 |
1Defined as 7 consecutive days with no heartburn reported in daily patient diary.
2Defined as the cumulative proportion of patients who have reached the start of sustained resolution
3log-rank test vs. omeprazole 20 mg
N.S. = not significant (p > 0.05).
In these four studies, the range of median days to the start of sustained resolution (defined as 7 consecutive days with no heartburn) was 5 days for esomeprazole magnesium 40 mg, 7 to 8 days for esomeprazole magnesium 20 mg and 7 to 9 days for omeprazole 20 mg.
There are no comparisons of 40 mg of esomeprazole magnesium with 40 mg of omeprazole in clinical trials assessing either healing or symptomatic relief of EE.
14.2 Maintenance of Healing of EE in Adults
Two multicenter, randomized, double-blind placebo-controlled 4-arm studies were conducted in adult patients with endoscopically confirmed, healed EE to evaluate esomeprazole magnesium delayed-release capsules 40 mg (n=174), 20 mg (n=180), 10 mg (n=168) or placebo (n=171) once daily over six months of treatment.
No additional clinical benefit was seen with esomeprazole magnesium 40 mg over esomeprazole magnesium 20 mg. Esomeprazole magnesium delayed-release capsules 40 mg once daily is not a recommended regimen for the maintenance of healing of EE in adults.
The percentages of patients that maintained healing of EE at the various time points are shown in the Figures 2 and 3:
Figure 2: Maintenance of Healing Rates of EE in Adults by Month (Study 177)
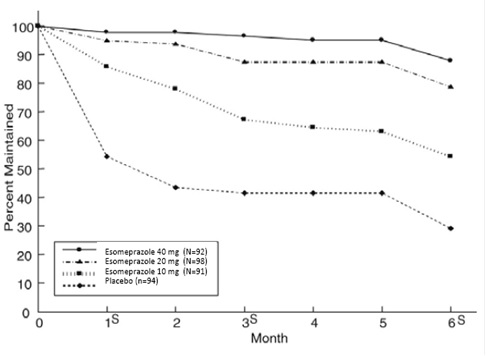
s= scheduled visit
Figure 3: Maintenance of EE Healing Rates in Adults by Month (Study 178)
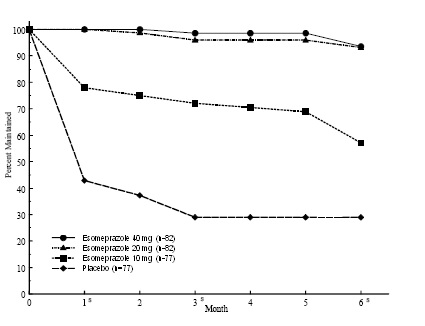
s= scheduled visit
Patients remained in remission significantly longer and the number of recurrences of EE was significantly less in patients treated with esomeprazole magnesium compared to placebo.
In both studies, the proportion of patients on esomeprazole magnesium who remained in remission and were free of heartburn and other GERD symptoms was well differentiated from placebo.
In a third multicenter open label study of 808 patients treated for 12 months with esomeprazole magnesium 40 mg, the percentage of patients that maintained healing of EE was 93.7% for six months and 89.4% for one year.
14.3 Symptomatic GERD in Adults
Two multicenter, randomized, double-blind, placebo-controlled studies were conducted in a total of 717 adult patients comparing four weeks of treatment with esomeprazole magnesium delayed-release capsules 20 mg or 40 mg once daily versus placebo for resolution of GERD symptoms. Patients had at least a 6-month history of heartburn episodes, no EE by endoscopy, and heartburn on at least four of the seven days immediately preceding randomization.
The percentage of patients that were symptom-free of heartburn was significantly higher in the esomeprazole magnesium groups compared to placebo at all follow-up visits (Weeks 1, 2, and 4).
No additional clinical benefit was seen with esomeprazole magnesium 40 mg over esomeprazole magnesium 20 mg. Esomeprazole magnesium 40 mg once daily is not a recommended regimen for the treatment of symptomatic GERD in adults.
The percent of patients symptom-free of heartburn by day are shown in the Figures 4 and 5:
Figure 4: Percent of Patients Symptom-Free of Heartburn by Day (Study 225)
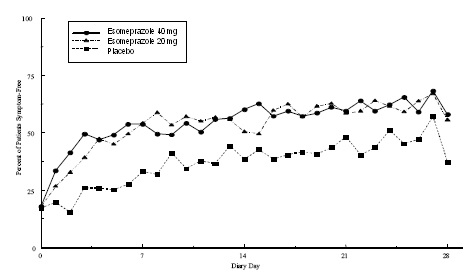
Figure 5: Pe****rcent of Patients Symptom-Free of Heartburn by Day (Study 226)
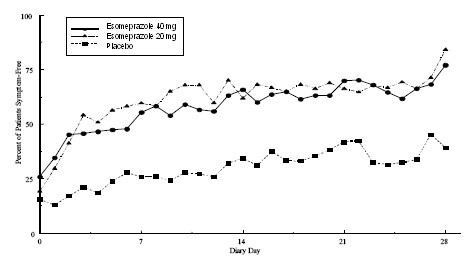
In three European symptomatic GERD trials, esomeprazole magnesium 20 mg and 40 mg and omeprazole 20 mg were evaluated. No significant treatment related differences were seen.
14.4 Pediatric GERD
12 Years to 17 Years of Age
In a multicenter, randomized, double-blind, parallel-group study, 149 adolescent patients (12 to 17 years of age; 89 female; 124 Caucasian, 15 Black, 10 Other) with clinically diagnosed GERD were treated with esomeprazole magnesium delayed-release capsules 20 mg or 40 mg once daily for up to 8 weeks to evaluate safety and tolerability. Patients were not endoscopically characterized as to the presence or absence of EE.
14.5 Risk Reduction of NSAID-Associated Gastric Ulcer
Two multicenter, double-blind, placebo-controlled studies were conducted in adult patients at risk of developing gastric and/or duodenal ulcers associated with continuous use of non-selective and COX-2 selective NSAIDs. A total of 1,429 patients were randomized across the 2 studies. Patients ranged in age from 19 to 89 (median age 66 years) with 71% female, 29% male, 83% Caucasian, 5% Black, 4% Asian, and 8% Others. At baseline, the patients in these studies were endoscopically confirmed not to have ulcers but were determined to be at risk for ulcer occurrence due to their age (at least 60 years) and/or history of a documented gastric or duodenal ulcer within the past 5 years. Patients receiving NSAIDs and treated with esomeprazole magnesium delayed-release capsules 20 mg or 40 mg once daily experienced significant reduction in gastric ulcer occurrences relative to placebo treatment at 26 weeks. See Table 13. No additional benefit was seen with esomeprazole magnesium 40 mg over esomeprazole magnesium 20 mg. Esomeprazole magnesium 40 mg once daily is not a recommended regimen for the risk reduction of NSAID-associated gastric ulcer in adults. These studies did not demonstrate significant reduction in the development of NSAID-associated duodenal ulcer due to the low incidence.
Table 13: Cumulative Percentage of Patients at Least 60 Years of Age Taking NSAIDS Without Gastric Ulcers at 26 Weeks in Two Randomized Placebo-Controlled Studies
|
Study |
No. of Patients |
Treatment Group |
% of Patients Remaining Gastric Ulcer Free1 |
|
1 |
191 194 184 |
Esomeprazole magnesium 20 mg Esomeprazole magnesium 40 mg Placebo |
95.4 96.7 88.2 |
|
2 |
267 271 257 |
Esomeprazole magnesium 20 mg Esomeprazole magnesium 40 mg Placebo |
94.7 95.3 83.3 |
1 %= Life Table Estimate. Significant difference from placebo (p<0.01).
14.6 H. pylori Eradication in Adult Patients with Duodenal Ulcer Disease
Two multicenter, randomized, double-blind studies were conducted in adult patients using a 10- day treatment regimen of triple therapy (esomeprazole magnesium, amoxicillin and clarithromycin). The first study (191) compared esomeprazole magnesium delayed-release capsules 40 mg once daily in combination with amoxicillin 1,000 mg twice daily and clarithromycin 500 mg twice daily to esomeprazole magnesium delayed-release capsules 40 mg once daily plus clarithromycin 500 mg twice daily. The second study (193) compared esomeprazole magnesium 40 mg once daily in combination with amoxicillin 1,000 mg twice daily and clarithromycin 500 mg twice daily to esomeprazole magnesium delayed-release capsules 40 mg once daily. H. pylori eradication rates, defined as at least two negative tests and no positive tests from CLOtest®, histology and/or culture, at 4 weeks post-therapy were significantly higher in the esomeprazole magnesium, amoxicillin and clarithromycin group than in the esomeprazole magnesium and clarithromycin group or the esomeprazole magnesium alone group. The results are shown in Table 14:
Table 14:H. pylori Eradication Rates at 4 Weeks after 10 Day Treatment Regimen % of Adult Patients Cured [95% Confidence Interval] (Number of Patients)
|
Study |
Treatment Group |
Per-Protocol1 |
Intent-to-Treat 2 |
|
191 |
Esomeprazole magnesium, amoxicillin and clarithromycin |
84%3 |
77% |
|
Esomeprazole magnesium, clarithromycin |
55% |
52% | |
|
193 |
Esomeprazole magnesium, amoxicillin and clarithromycin |
85% |
78%4 [67, 87] (n=74) |
|
Esomeprazole magnesium |
5% |
4% |
1. Patients were included in the analysis if they had H. pylori infection documented at baseline, had at least one endoscopically verified duodenal ulcer ≥ 0.5 cm in diameter at baseline or had a documented history of duodenal ulcer disease within the past 5 years, and were not protocol violators.
2. Patients who dropped out of the study due to an adverse reaction related to the study drug were included in the analysis as not H. pylori eradicated. Patients were included in the analysis if they had documented H. pylori infection at baseline, had at least one documented duodenal ulcer at baseline, or had a documented history of duodenal ulcer disease, and took at least one dose of study medication. All dropouts were included as not H. pylori eradicated.
3. p < 0.05 compared to esomeprazole magnesium plus clarithromycin.
4. p < 0.05 compared to esomeprazole magnesium alone.
The percentage of patients with a healed baseline duodenal ulcer by 4 weeks after the 10-day treatment regimen in the esomeprazole magnesium, amoxicillin and clarithromycin group was 75% (n=156) and 57% (n=60) respectively, in the 191 and 193 studies (per-protocol analysis).
14.7 Pathological Hypersecretory Conditions, Including Zollinger- Ellison
Syndrome, in Adults
In a multicenter, open-label dose-escalation study of 21 adult patients (15 males and 6 females, 18 Caucasian and 3 Black, mean age of 56 years) with pathological hypersecretory conditions, such as Zollinger-Ellison Syndrome, esomeprazole magnesium significantly inhibited gastric acid secretion. The initial dosage of esomeprazole magnesium delayed-release capsules was 40 mg twice daily in 19 patients and 80 mg twice daily in 2 patients. Total daily doses ranging from 80 mg to 240 mg for 12 months maintained gastric acid output below the target levels of 10 mEq/h in patients without prior gastric acid-reducing surgery and below 5 mEq/hr in patients with prior gastric acid- reducing surgery. At the Month 12 final visit, 18/20 (90%) patients had Basal Acid Output (BAO) under satisfactory control (median BAO = 0.17 mmol/hr). Of the 18 patients evaluated with a starting dose of esomeprazole magnesium 40 mg twice daily, 13 (72%) had their BAO controlled with the original dosing regimen at the final visit. See Table 15.
Table 15: Adequate Acid Suppression at Final Visit by Dosage Regimen in Adult Patients with Pathological Hypersecretory Conditions
|
Esomeprazole magnesium dose at the Month 12 visit |
**BAO under adequate control at the Month 12 visit (N=20)**1 |
|
40 mg twice daily |
13/15 |
|
80 mg twice daily |
4/4 |
|
80 mg three times daily |
1/1 |
1One patient was not evaluated.
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide and Instructions for Use).
Acute Tubulointerstitial Nephritis
Advise the patient or caregiver to call the patient’s healthcare provider immediately if they experience signs and/or symptoms associated with suspected acute TIN [see Warnings and Precautions (5.2)].
Clostridium difficile-Associated Diarrhea
Advise the patient or caregiver to immediately call the patient’s healthcare provider if they experience diarrhea that does not improve [see Warnings and Precautions (5.3)].
Bone Fracture
Advise the patient or caregiver to report any fractures, especially of the hip, wrist or spine, to the patient’s healthcare provider [see Warnings and Precautions (5.4)].
Severe Cutaneous Adverse Reactions
Advise the patient or caregiver to discontinue esomeprazole magnesium delayed- release capsules and immediately call the patient’s healthcare provider for at first appearance of a severe cutaneous adverse reaction or other sign of hypersensitivity signs or symptoms associated with Severe Cutaneous Adverse Reactions [see Warnings and Precautions (5.5)].
Cutaneous and Systemic Lupus Erythematosus
Advise the patient or caregiver to immediately call the patient’s healthcare provider for any new or worsening of symptoms associated with cutaneous or systemic lupus erythematosus [see Warnings and Precautions (5.6)].
Cyanocobalamin (Vitamin B-12) Deficiency
Advise the patient or caregiver to report any clinical symptoms that may be associated with cyanocobalamin deficiency to the patient’s healthcare provider if they have been receiving esomeprazole magnesium delayed-release capsules for longer than 3 years [see Warnings and Precautions (5.8)].
Hypomagnesemia and Mineral Metabolism
Advise the patient or caregiver to report any clinical symptoms that may be associated with hypomagnesemia, hypocalcemia, and/or hypokalemia to the patient’s healthcare provider, if they have been receiving esomeprazole magnesium delayed-release capsules for at least 3 months [see Warnings and Precautions (5.9)].
Drug Interactions
Advise the patient or caregiver to report to their healthcare provider if starting treatment with rilpivirine-containing products, clopidogrel, St. John’s Wort or rifampin; or, if they take high-dose methotrexate [see Contraindications (4), Warnings and Precautions (5.7, 5.10, 5.12)].
Administration
- Take esomeprazole magnesium delayed-release capsules at least one hour before meals.
- Antacids may be used concomitantly with esomeprazole magnesium delayed-release capsules.
- Swallow esomeprazole magnesium delayed-release capsules whole; do not chew or crush the capsules.
- For patients who have difficulty swallowing capsules, esomeprazole magnesium delayed-release capsules can be opened, and the contents sprinkled on applesauce. Use with other foods is not recommended.
1. Add one tablespoon of applesauce to an empty bowl. The applesauce used should not be hot and should be soft enough to be swallowed without chewing.
2. Open the esomeprazole magnesium delayed-release capsule and carefully empty the granules inside the capsule onto the applesauce.
3. Mix the granules with the applesauce.
4. Administer the mixture immediately. Do not chew or crush the granules
5. Discard any remaining mixture. Do not store the mixture for future use.
- Esomeprazole magnesium delayed-release capsules can also be administered via a nasogastric tube, as described in the Instructions for Use.
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage in Adults by Indication
Table 1 shows the recommended adult dosage of esomeprazole magnesium delayed- release capsules by indication.
The duration of esomeprazole magnesium delayed-release capsules treatment should be based on available safety and efficacy data specific to the defined indication and dosing frequency and individual patient medical needs. Esomeprazole magnesium delayed-release capsules should only be initiated and continued if the benefits outweigh the risks of treatment.
Table 1: Recommended Dosage of Esomeprazole Magnesium Delayed-Release Capsules in Adults by Indication
|
Adult Indication |
Recommended Dosage of Esomeprazole magnesium delayed-release capsules |
Treatment Duration |
|
Healing of EE |
20 mg or 40 mg1 once daily |
4 to 8 weeks2 |
|
Maintenance of Healing of EE |
20 mg once daily | |
|
Treatment of Symptomatic GERD |
20 mg once daily |
4 weeks; if symptoms do not resolve completely, consider an additional 4 weeks |
|
Risk Reduction of NSAID-Associated Gastric Ulcer |
20 mg or 40 mg1 once daily | |
|
H. pylori Eradication to Reduce the Risk of Duodenal Ulcer Recurrence (Triple Therapy) |
Esomeprazole 40 mg once daily1 |
10 days 10 days |
|
Pathological Hypersecretory Conditions Including Zollinger-Ellison Syndrome |
Dosages of up to 240 mg/day have been administered [see Clinical Studies (14.7)] |
As long as clinically indicated |
1. A maximum dosage of 20 mg once daily is recommended for patients with severe liver impairment (Child-Pugh Class C) [see Use in Specific Populations (8.6)].
2. Most patients are healed within 4 to 8 weeks. For patients who do not heal after 4 to 8 weeks, an additional 4 to 8 weeks of treatment may be required to achieve healing [see Clinical Studies (14.1)].
3. Refer to the amoxicillin and clarithromycin prescribing information for dosage adjustments in elderly and renally-impaired patients.
4. A starting dosage of 20 mg twice daily is recommended for patients with severe liver impairment (Child-Pugh Class C) [see Use in Specific Populations (8.6)].
2.2 Recommended Dosage in Pediatric Patients by Indication
Table 2 shows the recommended dosage of esomeprazole magnesium delayed-release capsules in pediatric patients by indication.
Table 2: Recommended Dosage of Esomeprazole Magnesium Delayed-Release Capsules in Pediatric Patients by Indication
|
Indication |
Patient Age |
Recommended Dosage |
Duration | |
|
Healing of EE |
12 years to 17 years |
Esomeprazole magnesium delayed-release capsules 20 mg or 40 mg once daily |
4 to 8 Weeks | |
|
Treatment of Symptomatic GERD |
12 years to 17 years |
Esomeprazole magnesium delayed-release capsules 20 mg once daily |
4 weeks |
1 Dosages over 1 mg/kg/day have not been studied
2 Dosages over 1.33 mg/kg/day have not been studied
2.3 Preparation and Administration Instructions
- Take esomeprazole magnesium delayed-release capsules at least one hour before meals [see Clinical Pharmacology (12.3)].
- Antacids may be used concomitantly with esomeprazole magnesium delayed-release capsules.
- Take a missed dose as soon as possible. If it is almost time for the next dose, skip the missed dose and take the next dose at the regular scheduled time. Do not take 2 doses at the same time.
Esomeprazole Magnesium Delayed-Release Capsules
Administer esomeprazole magnesium delayed-release capsules orally or via a nasogastric tube, as described below.
Oral Administration
- Swallow esomeprazole magnesium delayed-release capsules whole; do not chew or crush the capsules.
- For patients who have difficulty swallowing capsules, esomeprazole magnesium delayed-release capsules can be opened, and the contents sprinkled on applesauce. Use with other foods has not been evaluated and is not recommended.
1. Add one tablespoon of applesauce to an empty bowl. The applesauce used should not be hot and should be soft enough to be swallowed without chewing.
2. Open the esomeprazole magnesium delayed-release capsule and carefully empty the granules inside the capsule onto the applesauce.
3. Mix the granules with the applesauce.
4. Administer the mixture immediately. Do not chew or crush the granules
5. Discard any remaining mixture. Do not store the mixture for future use.
Administration via Nasogastric Tube
1. Open the esomeprazole magnesium delayed-release capsule and empty the granules into a 60 mL catheter-tipped syringe.
2. Mix the granules with 50 mL of water.
3. Replace the plunger and shake the catheter-tipped syringe vigorously for 15 seconds.
4. Hold the catheter-tipped syringe with the tip up and check for any granules remaining in the tip.
5. Attach the catheter-tipped syringe to a nasogastric tube and deliver the contents of the syringe through the nasogastric tube into the stomach.
6. After administering the granules, flush the nasogastric tube with additional water. Use the mixture immediately after preparation.
7. Do not administer the granules if they have dissolved or disintegrated.
|
Population |
** Recommended Adult (2.1) and Pediatric Dosage (2.2)** |
|
Healing of EE (1 year and older) | |
|
Adults |
20 mg or 40 mg1 once daily for 4 to 8 weeks; some patients may require an additional 4 to 8 weeks |
|
12 years to 17 years |
20 mg or 40 mg1 once daily for 4 to 8 weeks |
|
Maintenance of Healing of EE | |
|
Adults |
20 mg once daily. Controlled studies do not extend beyond 6 months |
|
Treatment of Symptomatic GERD | |
|
Adults |
20 mg once daily once daily for 4 weeks some patients may require an additional 4 weeks |
|
12 years to 17 years |
20 mg once daily for 4 weeks |
|
Risk Reduction of NSAID-Associated Gastric Ulcer | |
|
Adults |
20 mg or 40 mg1 once daily for up to 6 months2 |
|
H. pylori****Eradication to Reduce the Risk of Duodenal Ulcer Recurrence | |
|
Adults |
Esomeprazole magnesium delayed-release capsules 40 mg1 once daily for 10 days |
|
Pathological Hypersecretory Conditions Including Zollinger-Ellison Syndrome | |
|
Adults |
Starting dosage is 40 mg twice daily4 (varies with the individual patient) as long as clinically indicated. |
1 A maximum dosage of 20 mg once daily is recommended for patients with severe liver impairment (Child-Pugh Class C).
2 Controlled studies do not extend beyond 6 months.
3 Refer to the amoxicillin and clarithromycin prescribing information for dosage adjustments in elderly and renally-impaired patients.
4 A starting dosage of 20 mg twice daily is recommended for patients with severe liver impairment (Child-Pugh Class C).
Preparation and Administration Information
- Swallow capsules whole; do not crush or chew. For patients who cannot swallow intact capsule, the capsule can be opened, and the contents mixed with applesauce. (2.3)
- Opened capsules can be administered through a nasogastric tube. (2.3)
