QINLOCK
These highlights do not include all the information needed to use QINLOCK safely and effectively. See full prescribing information for QINLOCK. QINLOCK (ripretinib) tablets, for oral useInitial U.S. Approval: 2020
9f18e462-03dd-4296-a02a-a0577e3ee78d
HUMAN PRESCRIPTION DRUG LABEL
Oct 31, 2023
Deciphera Pharmaceuticals, LLC
DUNS: 078027928
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Ripretinib
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Principal Display Panel - 90 Tablet Carton Label
NDC: 73207-101-30Rx only
QINLOCK™
(ripretinib) tablets
50 mg
Dispense the enclosed Patient
Information Leaflet to each patient.
90
TABLETS
INDICATIONS & USAGE SECTION
1 INDICATIONS AND USAGE
QINLOCK is indicated for the treatment of adult patients with advanced gastrointestinal stromal tumor (GIST) who have received prior treatment with 3 or more kinase inhibitors, including imatinib.
QINLOCK is a kinase inhibitor indicated for the treatment of adult patients with advanced gastrointestinal stromal tumor (GIST) who have received prior treatment with 3 or more kinase inhibitors, including imatinib. (1)
WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Palmar-Plantar Erythrodysesthesia Syndrome
In INVICTUS, Grade 1-2 palmar-plantar erythrodysesthesia syndrome (PPES) occurred in 21% of the 85 patients who received QINLOCK [see Adverse Reactions (6.1)]. PPES led to dose discontinuation in 1.2% of patients, dose interruption in 2.4% of patients, and dose reduction in 1.2% of patients.
Based on severity, withhold QINLOCK and then resume at same or reduced dose [see Dosage and Administration (2.2)].
5.2 New Primary Cutaneous Malignancies
In INVICTUS, cutaneous squamous cell carcinoma (cuSCC) occurred in 4.7% of the 85 patients who received QINLOCK, with a median time to event of 4.6 months (range: 3.8 to 6 months). In the pooled safety population, cuSCC and keratoacanthoma occurred in 7% and 1.9% of patients, respectively.
In INVICTUS, melanoma occurred in 2.4% of the 85 of patients who received QINLOCK. In the pooled safety population, melanoma occurred in 0.9% of patients.
Perform dermatologic evaluations when initiating QINLOCK and routinely during treatment. Manage suspicious skin lesions with excision and dermatopathologic evaluation. Continue QINLOCK at the same dose.
5.3 Hypertension
In INVICTUS, Grade 1-3 hypertension occurred in 14% of the 85 patients who received QINLOCK, including Grade 3 hypertension in 7% [see Adverse Reactions (6.1)].
Do not initiate QINLOCK in patients with uncontrolled hypertension. Adequately control blood pressure prior to initiating QINLOCK. Monitor blood pressure as clinically indicated during treatment with QINLOCK, and initiate or adjust antihypertensive therapy as appropriate. Based on severity, withhold QINLOCK and then resume at same or reduced dose or permanently discontinue [see Dosage and Administration (2.2)].
5.4 Cardiac Dysfunction
In INVICTUS, cardiac failure occurred in 1.2% of the 85 patients who received QINLOCK. In the pooled safety population, cardiac dysfunction (including cardiac failure, acute left ventricular failure, diastolic dysfunction, and ventricular hypertrophy) occurred in 1.7% of patients, including Grade 3 adverse reactions in 1.1%.
In INVICTUS, Grade 3 decreased ejection fraction occurred in 1.3% of the 77 patients who received QINLOCK and who had a baseline and at least one post- baseline echocardiogram. In the pooled safety population, Grade 3 decreased ejection fraction occurred in 1.9% of the 263 patients who received QINLOCK and who had a baseline and at least one post-baseline echocardiogram.
In INVICTUS, cardiac dysfunction led to dose discontinuation in 1.2% of the 85 patients who received QINLOCK. The safety of QINLOCK has not been assessed in patients with a baseline ejection fraction below 50%.
Assess ejection fraction by echocardiogram or MUGA scan prior to initiating QINLOCK and during treatment, as clinically indicated. Permanently discontinue QINLOCK for Grade 3 or 4 left ventricular systolic dysfunction [see Dosage and Administration (2.2)].
5.5 Risk of Impaired Wound Healing
Impaired wound healing complications can occur in patients who receive drugs that inhibit the vascular endothelial growth factor (VEGF) signaling pathway. Therefore, QINLOCK has the potential to adversely affect wound healing.
Withhold QINLOCK for at least 1 week prior to elective surgery. Do not administer for at least 2 weeks following major surgery and until adequate wound healing. The safety of resumption of QINLOCK after resolution of wound healing complications has not been established.
5.6 Photosensitivity
QINLOCK may cause photosensitivity reactions. In all patients treated with QINLOCK in clinical trials (n=621), photosensitivity reactions occurred in 0.6% of patients.
Advise patients to limit direct ultraviolet exposure during treatment with QINLOCK and for at least one week after discontinuation of treatment.
5.7 Embryo-Fetal Toxicity
Based on findings from animal studies and its mechanism of action, QINLOCK can cause fetal harm when administered to a pregnant woman. Oral administration of ripretinib to pregnant rats and rabbits during the period of organogenesis resulted in malformations primarily associated with the cardiovascular and skeletal systems, anatomic variations, decreased fetal body weight, and increased post-implantation loss at exposures approximately one half of the recommended dose of 150 mg once daily based on area under the curve (AUC).
Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with QINLOCK and for 1 week after the last dose. Advise males with female partners of reproductive potential to use effective contraception during treatment with QINLOCK and for 1 week after the last dose [see Use in Specific Populations (8.1, 8.3), Nonclinical Toxicology (13.1)].
- Palmar-Plantar Erythrodysesthesia Syndrome: Based on severity, withhold QINLOCK and resume at same or reduced dose. (2.2, 5.1)
- New Primary Cutaneous Malignancies: Perform dermatologic evaluations when initiating QINLOCK and routinely during treatment. (5.2)
- Hypertension: Do not initiate QINLOCK in patients with uncontrolled hypertension and monitor blood pressure during treatment. Based on severity, withhold QINLOCK and then resume at same or reduced dose or permanently discontinue. (2.2, 5.3)
- Cardiac Dysfunction: Assess ejection fraction by echocardiogram or MUGA scan prior to initiating QINLOCK and during treatment, as clinically indicated. Permanently discontinue QINLOCK for Grade 3 or 4 left ventricular systolic dysfunction. (2.2, 5.4)
- Risk of Impaired Wound Healing: Withhold QINLOCK for at least 1 week prior to elective surgery. Do not administer for at least 2 weeks after major surgery and until adequate wound healing. The safety of resumption of QINLOCK after resolution of wound healing complications has not been established. (5.5)
- Photosensitivity: May cause photosensitivity reactions. Advise patients to limit direct ultraviolet exposure. (5.6)
- Embryo-Fetal Toxicity: Can cause fetal harm. Advise females of reproductive potential of the potential risk to a fetus and to use effective contraception. (5.7, 8.1, 8.3)
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
7.1 Effect of Other Drugs on QINLOCK
Table 4 includes drug interactions that affect the pharmacokinetics of ripretinib.
Table 4: Drug Interactions that Affect QINLOCK|
Strong CYP3A Inhibitors | |
|
Clinical Impact |
|
|
Prevention or Management |
|
|
Strong and Moderate CYP3A Inducers | |
|
Clinical Impact |
|
|
Prevention or Management |
|
- Strong CYP3A Inhibitors: Monitor more frequently for adverse reactions. (7.1)
- Strong CYP3A Inducers: Avoid concomitant use of strong CYP3A inducers. (7.1)
- Moderate CYP3A Inducers: Avoid concomitant use of moderate CYP3A inducers. If a moderate CYP inducer cannot be avoided, increase ripretinib dose frequency to twice daily. (2.3, 7.1)
DOSAGE FORMS & STRENGTHS SECTION
3 DOSAGE FORMS AND STRENGTHS
Tablets: 50 mg, white to off-white, oval shaped, debossed with “DC1” on one side.
Tablets: 50 mg. (3)
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
The efficacy of QINLOCK was evaluated in INVICTUS, an international, multi- center, randomized (2:1), double-blind, placebo-controlled trial (NCT03353753). Eligible patients had unresectable, locally advanced or metastatic gastrointestinal stromal tumor (GIST) and had received prior treatment with imatinib, sunitinib, and regorafenib. Randomization was stratified by prior lines of therapy (3 versus ≥4) and Eastern Cooperative Oncology Group (ECOG) performance status (0 versus 1 or 2). Patients received QINLOCK 150 mg or placebo orally once daily until disease progression or unacceptable toxicity. Tumor response assessments were performed every 28 days through for the first 4 months and then every 56 days thereafter. The major efficacy outcome measure was progression-free survival (PFS) based on disease assessment by blinded independent central review (BICR) using modified RECIST 1.1 criteria, in which lymph nodes and bone lesions were not target lesions and a progressively growing new tumor nodule within a pre-existing tumor mass must meet specific criteria to be considered unequivocal evidence of progression. Additional efficacy outcome measures included objective response rate (ORR) by BICR and overall survival (OS). Patients randomized to receive placebo could be treated with QINLOCK at the time of disease progression.
A total of 129 patients were randomized, 85 to QINLOCK and 44 to placebo.
Patient characteristics of the intent-to-treat (ITT) population in INVICTUS were median age of 60 years (range: 29 to 83 years), with 39% aged ≥65 years; 57% were male; 75% were White; and 92% had an ECOG performance status of 0 or
- Sixty-three percent (63%) of patients received 3 prior therapies and 37% received 4 or more prior therapies. Sixty-six percent (66%) of patients randomized to placebo switched to QINLOCK after disease progression.
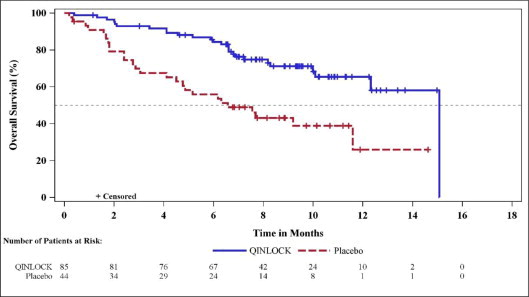
Efficacy results from INVICTUS are summarized in Table 6.
Table 6: Efficacy Results of INVICTUS|
BICR=Blinded Independent Central Review; CI=Confidence Interval | ||
|
a. Assessed per BICR. | ||
|
b. p-value is based on 2-sided stratified log-rank test. | ||
|
c. Hazard ratio is based on Cox proportional regression model. This model includes treatment and randomization stratification factors as fixed factors. | ||
|
d. Based on Fisher's exact test. The p-value is not statistically significant. | ||
|
e. Not evaluated for statistical significance as a result of the sequential testing procedure for the secondary endpoints of ORR and OS. | ||
|
QINLOCK |
Placebo | |
|
Progression-Free Survival****a | ||
|
Number of events (%) |
51 (60) |
37 (84) |
|
Progressive disease |
46 (54) |
32 (73) |
|
Deaths |
5 (6) |
5 (11) |
|
Median PFS (months) (95% CI) |
6.3 (4.6, 6.9) |
1.0 (0.9, 1.7) |
|
Hazard ratio (95% CI)c |
0.15 (0.09, 0.25) | |
|
p-valueb |
< 0.0001 | |
|
Overall Response Rate****a | ||
|
Overall Response Rate (%) |
9 |
0 |
|
(95% CI) |
(4.2, 18) |
(0, 8) |
|
p-valued |
0.0504 | |
|
Overall Survivale | ||
|
Number of deaths (%) |
26 (31) |
26 (59) |
|
Median OS (months) (95% CI) |
15.1 (12.3, 15.1) |
6.6 (4.1, 11.6) |
|
Hazard ratio (95% CI)c |
0.36 (0.21, 0.62) |
Figure 1: Kaplan-Meier Curve of Progression-Free Survival in INVICTUS
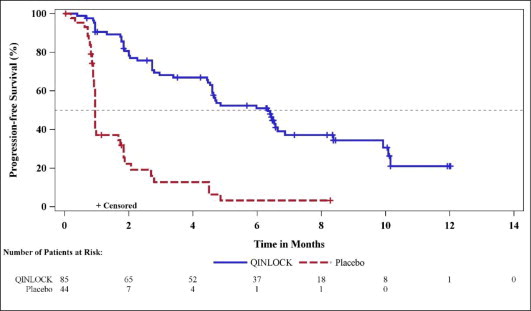
Figure 2: Kaplan-Meier Curve of Overall Survival in INVICTUS