Mirapex
These highlights do not include all the information needed to use MIRAPEX ER safely and effectively. See full prescribing information for MIRAPEX ER. MIRAPEX ER® (pramipexole dihydrochloride extended-release tablets), for oral use Initial U.S. Approval: 1997
e2902ed1-cfeb-4815-adc3-129c577917a1
HUMAN PRESCRIPTION DRUG LABEL
Nov 8, 2023
Boehringer Ingelheim Pharmaceuticals, Inc.
DUNS: 603175944
Products 7
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
pramipexole dihydrochloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (1)
pramipexole dihydrochloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (1)
pramipexole dihydrochloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (1)
pramipexole dihydrochloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (1)
pramipexole dihydrochloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (1)
pramipexole dihydrochloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (1)
pramipexole dihydrochloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (1)
Drug Labeling Information
USE IN SPECIFIC POPULATIONS SECTION
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no adequate data on the developmental risk associated with the use of MIRAPEX ER in pregnant women. No adverse developmental effects were observed in animal studies in which pramipexole was administered to rabbits during pregnancy. Effects on embryofetal development could not be adequately assessed in pregnant rats; however, postnatal growth was inhibited at clinically relevant exposures [see Data].
In the U.S. general population, the estimated background risk of major birth defects and of miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively. The background risk of major birth defects and miscarriage for the indicated population is unknown.
Data
Animal Data
Oral administration of pramipexole (0.1, 0.5, or 1.5 mg/kg/day) to pregnant rats during the period of organogenesis resulted in a high incidence of total resorption of embryos at the highest dose tested. This increase in embryolethality is thought to result from the prolactin-lowering effect of pramipexole; prolactin is necessary for implantation and maintenance of early pregnancy in rats but not in rabbits or humans. Because of pregnancy disruption and early embryonic loss in this study, the teratogenic potential of pramipexole could not be adequately assessed in rats. The highest no-effect dose for embryolethality in rats was associated with maternal plasma drug exposures (AUC) approximately equal to those in humans receiving the maximum recommended human dose (MRHD) of 4.5 mg/day. There were no adverse effects on embryo-fetal development following oral administration of pramipexole (0.1, 1, or 10 mg/kg/day) to pregnant rabbits during organogenesis (plasma AUC up to approximately 70 times that in humans at the MRHD). Postnatal growth was inhibited in the offspring of rats treated with pramipexole (0.1, 0.5, or 1.5 mg/kg/day) during the latter part of pregnancy and throughout lactation. The no-effect dose for adverse effects on offspring growth (0.1 mg/kg/day) was associated with maternal plasma drug exposures lower than that in humans at the MRHD.
8.2 Lactation
Risk Summary
There are no data on the presence of pramipexole in human milk, the effects of pramipexole on the breastfed infant, or the effects of pramipexole on milk production. However, inhibition of lactation is expected because pramipexole inhibits secretion of prolactin in humans. Pramipexole or metabolites, or both, are present in rat milk [see Data].
The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for MIRAPEX ER and any potential adverse effects on the breastfed infant from MIRAPEX ER or from the underlying maternal condition.
Data
In a study of radio-labeled pramipexole, pramipexole or metabolites, or both, were present in rat milk at concentrations three to six times higher than those in maternal plasma.
8.4 Pediatric Use
Safety and effectiveness of MIRAPEX ER tablets in pediatric patients have not been evaluated.
8.5 Geriatric Use
Pramipexole total oral clearance is approximately 30% lower in subjects older than 65 years compared with younger subjects, because of a decline in pramipexole renal clearance due to an age-related reduction in renal function. This resulted in an increase in elimination half-life from approximately 8.5 hours to 12 hours. In a placebo-controlled clinical trial of MIRAPEX ER tablets in early Parkinson's disease, 47% of the 259 patients were ≥65 years of age. Among patients receiving MIRAPEX ER tablets, hallucinations were more common in the elderly, occurring in 13% of the patients ≥65 years of age compared to 2% of the patients <65 years of age.
8.6 Renal Impairment
The elimination of pramipexole is dependent upon renal function. Pramipexole clearance is extremely low in dialysis patients, as a negligible amount of pramipexole is removed by dialysis [see Dosage and Administration (2.2), Warnings and Precautions (5.7), and Clinical Pharmacology (12.3)].
Pregnancy: Based on animal data, may cause fetal harm (8.1)
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Dosing Instructions
Instruct patients to take MIRAPEX ER tablets only as prescribed. If a dose is missed, MIRAPEX ER tablets should be taken as soon as possible, but no later than 12 hours after the regularly scheduled time. After 12 hours, the missed dose should be skipped and the next dose should be taken on the following day at the regularly scheduled time.
MIRAPEX ER tablets can be taken with or without food. If patients develop nausea, advise that taking MIRAPEX ER tablets with food may reduce the occurrence of nausea.
MIRAPEX ER tablets should be swallowed whole. They should not be chewed, crushed, or divided [see Dosage and Administration (2.1)].
Inform patients that residue in stool which may resemble a swollen original MIRAPEX ER tablet or swollen pieces of the original tablet have been reported [see Adverse Reactions (6.2)]. Instruct patients to contact their physician if this occurs.
Pramipexole is the active ingredient that is in both MIRAPEX ER tablets and immediate-release pramipexole tablets. Ensure that patients do not take both immediate-release pramipexole and MIRAPEX ER.
Sedating Effects
Alert patients to the potential sedating effects of MIRAPEX ER tablets, including somnolence and the possibility of falling asleep while engaged in activities of daily living. Since somnolence is a frequent adverse reaction with potentially serious consequences, patients should neither drive a car nor engage in other potentially dangerous activities until they have gained sufficient experience with MIRAPEX ER tablets to gauge whether or not it affects their mental and/or motor performance adversely. Advise patients that if increased somnolence or new episodes of falling asleep during activities of daily living (e.g., conversations or eating) are experienced at any time during treatment, they should not drive or participate in potentially dangerous activities until they have contacted their physician. Because of possible additive effects, advise caution when patients are taking other sedating medications or alcohol in combination with MIRAPEX ER and when taking concomitant medications that increase plasma levels of pramipexole (e.g., cimetidine) [see Warnings and Precautions (5.1)].
Postural (Orthostatic) Hypotension
Advise patients that they may develop postural (orthostatic) hypotension, with or without symptoms such as dizziness, nausea, fainting, or blackouts, and sometimes, sweating. Hypotension may occur more frequently during initial therapy. Accordingly, caution patients against rising rapidly after sitting or lying down, especially if they have been doing so for prolonged periods and especially at the initiation of treatment with MIRAPEX ER [see Warnings and Precautions (5.2)].
Impulse Control Symptoms Including Compulsive Behaviors
Alert patients and their caregivers to the possibility that they may experience intense urges to spend money, intense urges to gamble, increased sexual urges, binge eating and/or other intense urges and the inability to control these urges while taking MIRAPEX ER [see Warnings and Precautions (5.3)].
Hallucinations and Psychotic-like Behavior
Inform patients that hallucinations and other psychotic-like behavior can occur. In patients with Parkinson's disease, the elderly are at a higher risk than younger patients [see Warnings and Precautions (5.4)].
Withdrawal-Emergent Hyperpyrexia and Confusion
Advise patients who have been prescribed a lower dose or who have been withdrawn from the drug to notify their healthcare provider if they have symptoms such as fever, muscular rigidity, or altered consciousness [see Warnings and Precautions (5.10)].
Withdrawal Symptoms
Advise patients that withdrawal symptoms may occur during or after discontinuation or dose reduction of MIRAPEX ER. Advise patients who have been prescribed a lower dose or who have been withdrawn from the drug to notify their healthcare provider if they have withdrawal symptoms such as apathy, anxiety, depression, fatigue, insomnia, sweating, or pain. Notify patients that in case of severe withdrawal symptoms, a trial re-administration of a dopamine agonist at the lowest effective dose may be considered [see Warnings and Precautions (5.11)].
Pregnancy
Because the teratogenic potential of pramipexole has not been completely established in laboratory animals, and because experience in humans is limited, advise women to notify their physicians if they become pregnant or intend to become pregnant during therapy [see Use in Specific Populations (8.1)].
Lactation
Because of the possibility that pramipexole may be excreted in breast milk, advise women to notify their physicians if they intend to breast-feed or are breast-feeding an infant [see Use in Specific Populations (8.2)].
SPL PATIENT PACKAGE INSERT SECTION
Patient Information
MIRAPEX ER® (mîr'-ah-pěx)
(pramipexole dihydrochloride
extended-release tablets)
Read this Patient Information before you start taking MIRAPEX ER and each time you get a refill. There may be new information. This information does not take the place of talking with your doctor about your medical condition or your treatment.
What is MIRAPEX ER?
MIRAPEX ER is a prescription medicine used to treat the signs and symptoms of Parkinson's disease.
It is not known if MIRAPEX ER is safe and effective in children.
What should I tell my doctor before taking MIRAPEX ER?
Before taking MIRAPEX ER, tell your doctor if you:
- feel sleepy during the day
- have low blood pressure, or if you feel dizzy or faint, especially when getting up from sitting or lying down.
- have trouble controlling your muscles (dyskinesia)
- have kidney problems
- drink alcohol. Alcohol can increase the chance that MIRAPEX ER will make you feel sleepy or fall asleep when you should be awake.
- are pregnant or plan to become pregnant. It is not known if MIRAPEX ER will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if MIRAPEX ER passes into your breast milk. You and your doctor should decide if you will take MIRAPEX ER or breastfeed. You should not do both.
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
MIRAPEX ER and other medicines may affect each other causing side effects. MIRAPEX ER may affect the way other medicines work, and other medicines may affect how MIRAPEX ER works.
Especially tell your doctor if you take:
- medicines called neuroleptics (phenothiazines, butyrophenones, thioxanthenes) or metoclopramide. MIRAPEX ER may not work as well if you take these medicines.
- pramipexole (MIRAPEX). Pramipexole is the active ingredient in both MIRAPEX ER and MIRAPEX. If you are taking MIRAPEX, you should not take MIRAPEX ER.
- any other medicines that make you sleepy or may increase the effects of MIRAPEX ER, such as cimetidine (Tagamet).
Ask your doctor for a list of these medicines if you are not sure.
Know the medicines you take. Keep a list of them and show it to your doctor and pharmacist when you get a new medicine.
How should I take MIRAPEX ER?
- MIRAPEX ER is taken once daily.
- Your doctor will tell you how much MIRAPEX ER to take and when to take it. Do not take more or less MIRAPEX ER than your doctor tells you to.
- Swallow MIRAPEX ER whole. Do not chew, crush, or cut MIRAPEX ER.
- MIRAPEX ER can be taken with or without food. Taking MIRAPEX ER with food may lower your chances of getting nausea.
- You may see something that looks like a swollen original tablet or swollen pieces of the original tablet in your stool. If this happens, tell your doctor.
- If you miss a dose of MIRAPEX ER it should be taken as soon as possible, but no later than 12 hours after your regularly scheduled time. If it is later than 12 hours, the missed dose should be skipped and the next dose should be taken on the following day at your regularly scheduled time.Do not double your next MIRAPEX ER dose. *Do not stop taking MIRAPEX ER without talking to your doctor first. If your doctor tells you to stop taking MIRAPEX ER, you should ask your doctor for specific instructions on how to slowly and safely discontinue taking MIRAPEX ER. If you stop taking MIRAPEX ER you may have withdrawal symptoms (see "withdrawal symptoms" under "What are the possible side effects of MIRAPEX ER?").
What should I avoid while taking MIRAPEX ER?
- Do not drink alcohol while taking MIRAPEX ER. It can increase your chance of having serious side effects. See "What are the possible side effects of MIRAPEX ER?"
- Do not drive a car, operate a machine, or do other dangerous activities until you know how MIRAPEX ER affects you. Sleepiness caused by MIRAPEX ER can happen as late as 1 year after you start your treatment.
What are the possible side effects of MIRAPEX ER?
MIRAPEX ER may cause serious side effects, including:
*falling asleep during normal daily activities. MIRAPEX ER may cause you to fall asleep while you are doing daily activities such as driving, talking with other people, or eating. * Some people taking the medicine in MIRAPEX ER have had car accidents because they fell asleep while driving. * Some people did not feel sleepy before they fell asleep while driving. You could fall asleep without any warning. Tell your doctor right away if you fall asleep while you are doing activities such as talking, eating, driving, or if you feel sleepier than normal for you.
*low blood pressure when you sit or stand up quickly. After you have been sitting or lying down, stand up slowly until you know how MIRAPEX ER affects you. This may help reduce the following symptoms while you are taking MIRAPEX ER:
* dizziness
* nausea
* fainting
* sweating
*unusual urges. Some people who take certain medicines to treat Parkinson's disease, including MIRAPEX ER, have reported problems, such as gambling, compulsive eating, compulsive buying, and increased sex drive.
If you or your family members notice that you are developing unusual urges or
behaviors, talk to your doctor.
*hallucinations and other psychotic-like behavior (seeing visions, hearing sounds or feeling sensations that are not real, confusion, excessive suspicion, aggressive behavior, agitation, delusional beliefs and disorganized thinking). The chances of having hallucinations or other psychotic-like changes are higher in people taking MIRAPEX ER for Parkinson's disease who are elderly (age 65 or older).
If you have hallucinations or other psychotic-like changes, talk with your
doctor right away.
*uncontrolled sudden movements (dyskinesia). If you have new dyskinesia, or your existing dyskinesia gets worse, tell your doctor. *posture changes. Talk with your doctor if you have posture changes you cannot control. These may include your neck bending forward, bending forward at the waist, or tilting sideways when you sit, stand, or walk. *withdrawal symptoms. MIRAPEX ER is a dopamine agonist medicine. Dopamine agonist medicines, including MIRAPEX ER, can cause withdrawal symptoms as your dose is slowly lowered (tapered) or when treatment with MIRAPEX ER is stopped. Tell your doctor right away if you get any of the following withdrawal symptoms: * fever * confusion * severe muscle stiffness * feeling like you do not care about things you usually care about (apathy) * anxiety * depression * fatigue * insomnia * sweating * pain
After you have stopped taking MIRAPEX ER, your doctor may need to restart you at a low dose of MIRAPEX ER if you get severe withdrawal symptoms.
The most common side effects in people taking MIRAPEX ER for early Parkinson's disease are:
- nausea and vomiting
- constipation
- dizziness
- fatigue
- dry mouth
- swelling of the feet and ankles
The most common side effects in people taking MIRAPEX ER who have later stage Parkinson's disease are nausea, constipation, headache and weight loss (anorexia).
These are not all the possible side effects of MIRAPEX ER. Tell your doctor if you have any side effect that bothers you.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store MIRAPEX ER?
- Store MIRAPEX ER at room temperature from 68°F to 77°F (20°C to 25°C).
- Keep MIRAPEX ER away from high humidity or moisture.
- Keep MIRAPEX ER and all medicines out of the reach of children.
General Information about the safe and effective use of MIRAPEX ER.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use MIRAPEX ER for a condition for which it was not prescribed. Do not give MIRAPEX ER to other people, even if they have the same symptoms that you have. It may harm them.
This Patient Information leaflet summarizes the most important information about MIRAPEX ER. If you would like more information, talk with your doctor. You can ask your pharmacist or doctor for more information about MIRAPEX ER tablets that is written for healthcare professionals.
For current Prescribing Information, scan the code or for additional information, you may also call Boehringer Ingelheim Pharmaceuticals, Inc. at 1-800-542-6257.

What are the ingredients in MIRAPEX ER?
Active Ingredient: pramipexole dihydrochloride monohydrate.
Inactive Ingredients: hypromellose, corn starch, carbomer homopolymer, colloidal silicon dioxide, and magnesium stearate.
What does MIRAPEX ER look like?
These pictures show what MIRAPEX ER tablets look like. Notice that each strength tablet looks different. Immediately call your pharmacist if you receive a MIRAPEX ER tablet that does not look like one of the tablets shown below, as you may have received the wrong medication.
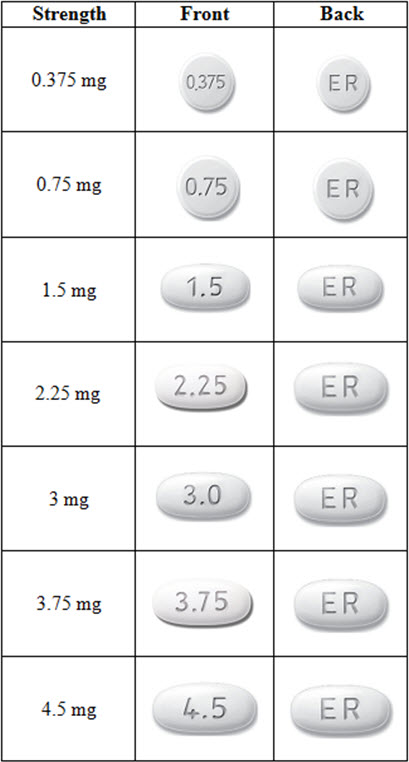
Tablets not actual size.
This Patient Information has been approved by the U.S. Food and Drug Administration.
Distributed by:
Boehringer Ingelheim Pharmaceuticals, Inc.
Ridgefield, CT 06877 USA
Licensed from:
Boehringer Ingelheim International GmbH
MIRAPEX is a registered trademark of and used under license from Boehringer Ingelheim International GmbH.
Copyright © 2021 Boehringer Ingelheim International GmbH
ALL RIGHTS RESERVED
Revised: 7/2021
COL9694AG162021
