Digoxin
These highlights do not include all the information needed to use DIGOXIN TABLETS safely and effectively. See full prescribing information for DIGOXIN TABLETS. DIGOXIN tablets, for oral use Initial U.S. Approval: 1954
e104c3c5-e4b8-7dbd-e053-2995a90ae339
HUMAN PRESCRIPTION DRUG LABEL
Feb 28, 2023
Marlex Pharmaceuticals, Inc.
DUNS: 782540215
Products 2
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Digoxin
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
Digoxin
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (5)
Drug Labeling Information
INDICATIONS & USAGE SECTION
1 INDICATIONS & USAGE
1.1 Heart Failure in Adults
Digoxin is indicated for the treatment of mild to moderate heart failure in adults. Digoxin increases left ventricular ejection fraction and improves heart failure symptoms as evidenced by improved exercise capacity and decreased heart failure-related hospitalizations and emergency care, while having no effect on mortality. Where possible, Digoxin should be used in combination with a diuretic and an angiotensin-converting enzyme (ACE) inhibitor.
1.2 Heart Failure in Pediatric Patients
Digoxin increases myocardial contractility in pediatric patients with heart failure.
1.3 Atrial Fibrillation in Adults
Digoxin is indicated for the control of ventricular response rate in adult patients with chronic atrial fibrillation.
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis & Mutagenesis & Impairment Of Fertility
Digoxin showed no genotoxic potential in in vitro studies (Ames test and mouse lymphoma). No data are available on the carcinogenic potential of digoxin, nor have studies been conducted to assess its potential to affect fertility.
USE IN SPECIFIC POPULATIONS SECTION
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Experience with digoxin in pregnant women over several decades, based on published retrospective clinical studies and case reports, has not led to the identification of a drug associated risk of major birth defects, miscarriage or adverse maternal and fetal outcomes. Untreated underlying maternal conditions, such as heart failure and atrial fibrillation, during pregnancy pose a risk to mother and fetus ( see clinical consideration). Animal reproduction studies have not been conducted with digoxin.
The estimated background risk of major birth defects and miscarriage for the indicated population(s) are unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2%-4% and 15%-20%, respectively.
Clinical Considerations
Disease-associated maternal and/or embryo/fetal risk
Pregnant women with heart failure are at increased risk for preterm birth. Clinical classification of heart disease may worsen with pregnancy and lead to maternal or fetal death.
Pregnant women with atrial fibrillation are at increased risk of delivering a low birth weight infant. Atrial fibrillation may worsen with pregnancy and can lead to maternal or fetal death.
Fetal/neonatal adverse reactions
Digoxin has been shown to cross the placenta and is found in amniotic fluid. Monitor neonates for signs and symptoms of digoxin toxicity, including vomiting, cardiac arrhythmias [see Warnings and Precautions (5.3)].
Dose adjustments during pregnancy and the postpartum period
Digoxin requirements may increase during pregnancy and decrease in the postpartum period. Monitor serum digoxin levels during pregnancy and postpartum period [see Dosage and Administration (2.5)].
Labor or Delivery
Risk of arrhythmias may increase during labor and delivery. Monitor patients continuously during labor and delivery [see Warnings and Precautions (5.1 and 5.2)].
8.2 Lactation
Risk Summary
The digoxin dose received through breastfeeding is up to 4% of the neonatal maintenance dosage, which is unlikely to be clinically relevant. There are no data on the effects of digoxin on the breastfed infant or effects on milk production.
Data
Based on data from two lactation studies in a total of 13 breastfed infants, the digoxin concentrations in breast milk were between 0.4 - 1.0 ng/ml following 0.25 mg once daily dose of digoxin in the lactating mother. Thus, the amount of digoxin ingested daily by the infants is estimated to be between 0.03 to 0.16 μg/kg/day. This translates to relative infant dose of digoxin between 1 to 7% of maternal weight- adjusted dose and about 0.2 to 4% of the neonatal maintenance dose.
8.4 Pediatric Use
The safety and effectiveness of Digoxin in the control of ventricular rate in children with atrial fibrillation have not been established.
The safety and effectiveness of Digoxin in the treatment of heart failure in children have not been established in adequate and well-controlled studies. However, in published literature of children with heart failure of various etiologies (e.g., ventricular septal defects, anthracycline toxicity, patent ductus arteriosus), treatment with digoxin has been associated with improvements in hemodynamic parameters and in clinical signs and symptoms.
Newborn infants display considerable variability in their tolerance to digoxin. Premature and immature infants are particularly sensitive to the effects of digoxin, and the dosage of the drug must not only be reduced but must be individualized according to their degree of maturity.
8.5 Geriatric Use
The majority of clinical experience gained with digoxin has been in the elderly population. This experience has not identified differences in response or adverse effects between the elderly and younger patients. However, this drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, which should be based on renal function, and it may be useful to monitor renal function [see Dosage and Administration (2.1)].
8.6 Renal Impairment
The clearance of digoxin can be primarily correlated with the renal function as indicated by creatinine clearance. Tables 3 and 5 provide the usual daily maintenance dose requirements for digoxin based on creatinine clearance [see Dosage and Administration (2.3)].
Digoxin is primarily excreted by the kidneys; therefore, patients with impaired renal function require smaller than usual maintenance doses of digoxin [see Dosage and Administration (2.3)]. Because of the prolonged elimination half-life, a longer period of time is required to achieve an initial or new steady- state serum concentration in patients with renal impairment than in patients with normal renal function. If appropriate care is not taken to reduce the dose of digoxin, such patients are at high risk for toxicity, and toxic effects will last longer in such patients than in patients with normal renal function.
8.7 Hepatic Impairment
Plasma digoxin concentrations in patients with acute hepatitis generally fall within the range of profiles in a group of healthy subjects.
8.8 Malabsorption
The absorption of digoxin is reduced in some malabsorption conditions such as chronic diarrhea.
- Pregnant patients: It is unknown whether use during pregnancy can cause fetal harm. (8.1)
- Pediatric patients: Newborn infants display variability in tolerance to Digoxin. (8.4)
- Geriatric patients: Consider renal function in dosage selection, and carefully monitor for side effects. (8.5)
- Renal impairment: Digoxin is excreted by the kidneys. Consider renal function during dosage selection. (8.6)
(8)
(8)
(8)
DESCRIPTION SECTION
11 DESCRIPTION
Digoxin is one of the cardiac (or digitalis) glycosides, a closely related group of drugs having in common specific effects on the myocardium. These drugs are found in a number of plants. Digoxin is extracted from the leaves of Digitalis lanata. The term “digitalis” is used to designate the whole group of glycosides. The glycosides are composed of 2 portions: a sugar and a cardenolide (hence “glycosides”).
Digoxin is described chemically as (3β,5β,12β)-3-[( O-2,6-dideoxy-β- D-ribo- hexopyranosyl-(1→4)- O- 2,6-dideoxy-β- D-ribo- hexopyranosyl-(1→4)-2,6-dideoxy-β- D- ribo-hexopyranosyl)oxy]-12,14- dihydroxy-card-20(22)-enolide. Its molecular formula is C41H64O14, its molecular weight is 780.95, and its structural formula is:
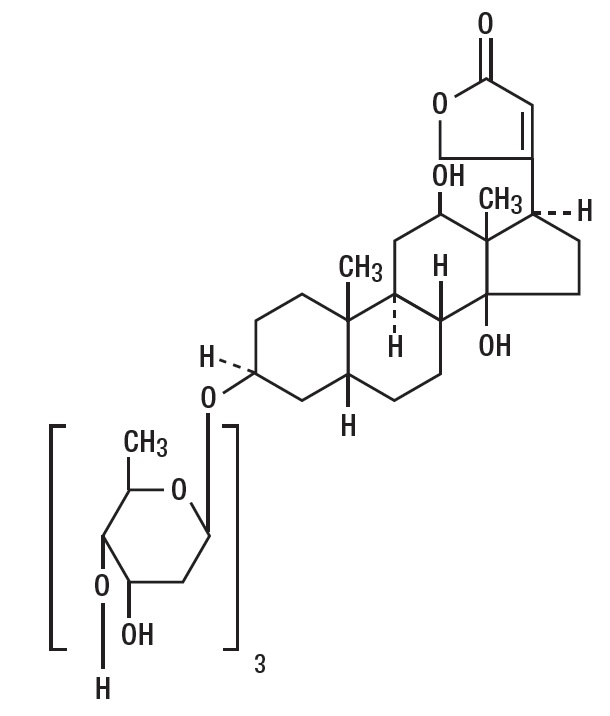
Digoxin exists as white or almost white powder or colorless crystals. The drug is practically insoluble in water; soluble in mixture of methanol & methylene chloride and slightly soluble in ethyl alcohol.
Digoxin is supplied as, 125 mcg (scored), and 250 mcg (scored) tablets for oral administration. Each tablet contains the labeled amount of digoxin USP and the following inactive ingredients: microcrystalline cellulose, pregelatinized starch, lactose anhydrous, magnesium stearate. The 125 mcg tablets additionally contain yellow iron oxide.
FDA approved dissolution test specifications differ from USP.
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
14.1 Chronic Heart Failure
Two 12-week, double-blind, placebo-controlled studies enrolled 178 (RADIANCE trial) and 88 (PROVED trial) adult patients with NYHA Class II or III heart failure previously treated with oral digoxin, a diuretic, and an ACE inhibitor (RADIANCE only) and randomized them to placebo or treatment with Digoxin Tablets. Both trials demonstrated better preservation of exercise capacity in patients randomized to Digoxin. Continued treatment with Digoxin reduced the risk of developing worsening heart failure, as evidenced by heart failure- related hospitalizations and emergency care and the need for concomitant heart failure therapy.
DIG Trial of Digoxin in Patients with Heart Failure
The Digitalis Investigation Group (DIG) main trial was a 37-week, multicenter, randomized, double- blind mortality study comparing digoxin to placebo in 6800 adult patients with heart failure and left ventricular ejection fraction less than or equal to 0.45. At randomization, 67% were NYHA class I or II, 71% had heart failure of ischemic etiology, 44% had been receiving digoxin, and most were receiving a concomitant ACE inhibitor (94%) and diuretics (82%). As in the smaller trials described above, patients who had been receiving open-label digoxin were withdrawn from this treatment before randomization. Randomization to digoxin was again associated with a significant reduction in the incidence of hospitalization, whether scored as number of hospitalizations for heart failure (relative risk 75%), risk of having at least one such hospitalization during the trial (RR 72%), or number of hospitalizations for any cause (RR 94%). On the other hand, randomization to digoxin had no apparent effect on mortality (RR 99%, with confidence limits of 91-107%).
14.2 Chronic Atrial Fibrillation
Digoxin has also been studied as a means of controlling the ventricular response to chronic atrial fibrillation in adults. Digoxin reduced the resting heart rate, but not the heart rate during exercise.
In 3 different randomized, double-blind trials that included a total of 315 adult patients, digoxin was compared to placebo for the conversion of recent- onset atrial fibrillation to sinus rhythm. Conversion was equally likely, and equally rapid, in the digoxin and placebo groups. In a randomized 120-patient trial comparing digoxin, sotalol, and amiodarone, patients randomized to digoxin had the lowest incidence of conversion to sinus rhythm, and the least satisfactory rate control when conversion did not occur.
In at least one study, digoxin was studied as a means of delaying reversion to atrial fibrillation in adult patients with frequent recurrence of this arrhythmia. This was a randomized, double-blind, 43-patient crossover study. Digoxin increased the mean time between symptomatic recurrent episodes by 54%, but had no effect on the frequency of fibrillatory episodes seen during continuous electrocardiographic monitoring.
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
Digoxin Tablets have "N" on one side and are supplied as follows:
Scored Tablets: 125 mcg are yellow, circular, beveled, uncoated tablets scored between "N" and "201" on one side and plain on the other side.
Bottles of 100ct (NDC 10135-0747-01)
Bottles of 1000ct (NDC 10135-0747-10)
Scored Tablets: 250 mcg are white to off-white, circular. beveled, uncoated tablets scored between "N" and "202" on one side and plain on the otherside.
Bottles of 100ct (NDC 10135-0748-01)
Bottles of 1000ct (NDC 10135-748-10)
Store at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F) [See USP Controlled Room Temperature] in a dry place and protect from light.
Dispense in tight, light-resistant container as defined in USP.
Keep this and all medication out of the reach of children.
