Respiratory Health Formula
Respiratory Health Formula
c273ad8e-a6da-4ffa-a51d-b4cd3371a7f8
OTC ANIMAL DRUG LABEL
Aug 12, 2025
Vsf2 Inc.
DUNS: 034543894
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Ammonium carbonicum, Antimonium arsenicicum, Antimonium tartaricum, Arsenicum album, Bromium, Carbo vegetabilis, Chlorinum, Drosera, Dulcamara, Kali carbonicum, Lobelia inflata, Stannum metallicum, Sulphuricum acidum, Phosphorus, and Spongia.
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (18)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
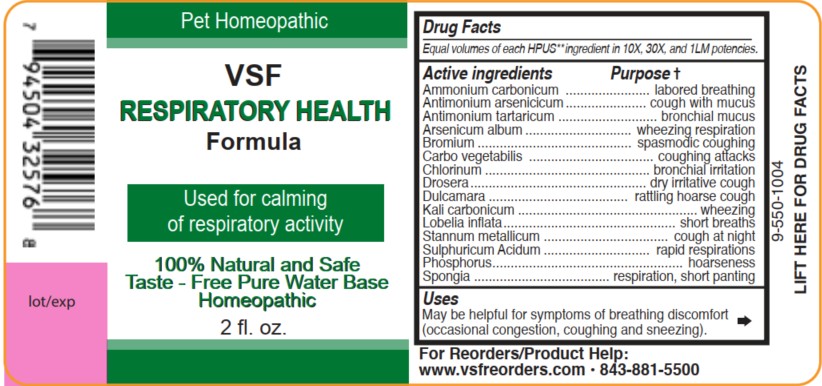
INDICATIONS & USAGE SECTION
Uses
May be helpful for symptoms of breathing discomfort (occasional congestion, coughing and sneezing).
OTC - QUESTIONS SECTION
Questions
VSF Respiratory Health Formula 2fl oz.
100% Natural and Safe Taste
Free Pure Water Base
Pet Homeopathic
For reorders/Product Help:
www.vsfreorders.com 843-881-5500
WARNINGS SECTION
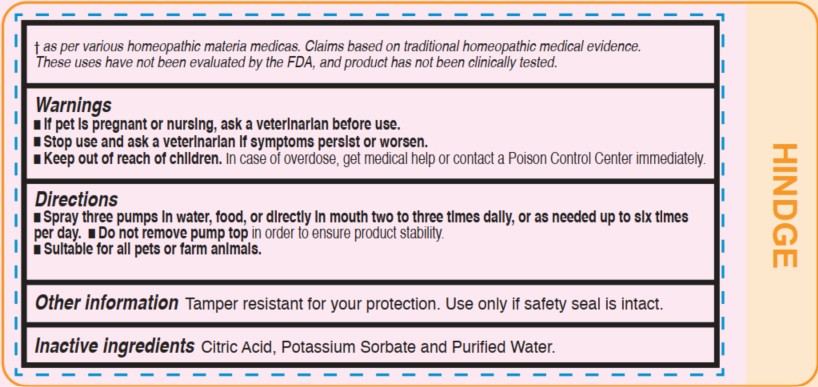
Warnings
- If pet is pregnant or nursing, ask a veterinarian before use.
- Stop use and ask a veterinarian if symptoms persist or worsen.
- Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center immediately.
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center immediately.
DOSAGE & ADMINISTRATION SECTION
Directions
- Spray three pumps in water, food, or directly in mouth two to three times daily, or as needed up to six times per day.
- Do not remove pump top in order to ensure product stability.
- Suitable for all pets or farm animals.
OTHER SAFETY INFORMATION
Other information
Tamper resistant for your protection.
Use only if safety seal is intact.
OTC - ACTIVE INGREDIENT SECTION
Active ingredients
Equal volumes of each HPUS** ingredient in 10X,
30X, and 1LM potencies.
Ammonium carbonicum, Antimonium arsenicicum, Antimonium tartaricum, Arsenicum album, Bromium, Carbo vegetabilis, Chlorinum, Drosera, Dulcamara, Kali carbonicum, Lobelia inflata, Stannum metallicum, Sulphuricum acidum, Phosphorus, and Spongia.
As per various homeopathic materia medicas. Claims based on traditional homeopathic medical evidence.
These uses have not been evaluated by the FDA, and product has not been clinically tested.
INACTIVE INGREDIENT SECTION
Inactive Ingredients
Citric Acid, Potassium Sorbate, and Purified Water.
OTC - PURPOSE SECTION
Equal volumes of each HPUS** ingredient in 10X,
30X, and 1LM potencies.
Active ingredients Purpose †
Ammonium carbonicum.................................labored breathing
Antimonium arsenicicum...............................cough with mucus
Antimonium tartaricum...................................bronchial mucus
Arsenicum album.....................................wheezing respiration
Bromium.................................................spasmodic coughing
Carbo vegetabilis..........................................coughing attacks
Chlorinum..................................................bronchial irritation
Drosera....................................................dry irritative cough
Dulcamara.............................................rattling hoarse cough
Kali carbonicum......................................................wheezing
Lobelia inflata...................................................short breaths
Stannum metallicum........................................cough at night
Sulphuricum acidum....................................rapid respirations
Phosphorus.........................................................hoarseness
Spongia..........................................respiration, short panting
As per various homeopathic materia medicas. Claims based on traditional homeopathic medical evidence.
These uses have not been evaluated by the FDA, and product has not been clinically tested.
