LEXIVA
These highlights do not include all the information needed to use LEXIVA safely and effectively. See full prescribing information for LEXIVA.LEXIVA (fosamprenavir calcium) Tablets and Oral SuspensionInitial U.S. Approval: 2003
009575f9-74e8-4a6d-9fa6-3cae72fd01c3
HUMAN PRESCRIPTION DRUG LABEL
Apr 14, 2010
State of Florida DOH Central Pharmacy
DUNS: 829348114
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
fosamprenavir calcium
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (10)
Drug Labeling Information
SPL PATIENT PACKAGE INSERT SECTION
PATIENT INFORMATION
LEXIVA**®**
(lex-EE-vah)
(fosamprenavir calcium)
Tablets and Oral Suspension
Read the Patient Information that comes with LEXIVA before you start taking it and each time you get a refill. There may be new information. This information does not take the place of talking with your healthcare provider about your medical condition or treatment. It is important to remain under a healthcare provider's care while taking LEXIVA. Do not change or stop treatment without first talking with your healthcare provider. Talk to your healthcare provider or pharmacist if you have any questions about LEXIVA.
What is the most important information I should know about LEXIVA?
LEXIVA can cause dangerous and life-threatening interactions if taken with certain other medicines. Tell your healthcare provider about all the medicines you take, including prescription and nonprescription medicines, vitamins, and herbal supplements.
- Some medicines cannot be taken at all with LEXIVA.
- Some medicines will require dose changes if taken with LEXIVA.
- Some medicines will require close monitoring if you take them with LEXIVA.
Know all the medicines you take, including prescription and nonprescription medicines, vitamins, and herbal supplements. Keep a list of the medicines you take. Show this list to all your healthcare providers and pharmacists anytime you get a new medicine or refill. Your healthcare providers and pharmacists must know all the medicines you take. They will tell you if you can take other medicines with LEXIVA. Do not start any new medicines while you are taking LEXIVA without talking with your healthcare provider or pharmacist. You can ask your healthcare provider or pharmacist for a list of medicines that can interact with LEXIVA.
What is LEXIVA?
LEXIVA is a medicine you take by mouth to treat HIV infection. HIV is the virus that causes AIDS (acquired immune deficiency syndrome). LEXIVA belongs to a class of anti-HIV medicines called protease inhibitors. LEXIVA is always used with other anti-HIV medicines. When used in combination therapy, LEXIVA may help lower the amount of HIV found in your blood, raise CD4+ (T) cell counts, and keep your immune system as healthy as possible, so it can help fight infection. However, LEXIVA does not work in all patients with HIV.
LEXIVA does not:
- cure HIV infection or AIDS. We do not know if LEXIVA will help you live longer or have fewer of the medical problems (opportunistic infections) that people get with HIV or AIDS. Opportunistic infections are infections that develop because the immune system is weak. Some of these conditions are pneumonia, herpes virus infections, and Mycobacterium avium complex (MAC) infections. It is very important that you see your healthcare provider regularly while you are taking LEXIVA. The long-term effects of LEXIVA are not known.
- lower the risk of passing HIV to other people through sexual contact, sharing needles, or being exposed to your blood. For your health and the health of others, it is important to always practice safer sex by using a latex or polyurethane condom to lower the chance of sexual contact with semen, vaginal secretions, or blood. Never use or share dirty needles.
LEXIVA has not been fully studied in children under the age of 2 or in adults over the age of 65.
Who should not take LEXIVA?
Do not take LEXIVA if you:
- are taking certain other medicines. Read the section“What is the most important information I should know about LEXIVA?” Do not take the following medicines* with LEXIVA. You could develop serious or life-threatening problems.
- HALCION® (triazolam; used for insomnia)
- Ergot medicines: dihydroergotamine, ergonovine, ergotamine, and methylergonovine such as CAFERGOT®, MIGRANAL®, D.H.E. 45®, ergotrate maleate, METHERGINE®, and others (used for migraine headaches)
- PROPULSID® (cisapride), used for certain stomach problems
- VERSED® (midazolam), used for sedation
- ORAP® (pimozide), used for Tourette’s disorder
- are allergic to LEXIVA or any of its ingredients. The active ingredient is fosamprenavir calcium. See the end of this leaflet for a list of all the ingredients in LEXIVA.
- are allergic to AGENERASE (amprenavir).
You should not take AGENERASE (amprenavir) and LEXIVA at the same time.
There are other medicines you should not take if you are taking LEXIVA and NORVIR® (ritonavir) together. You could develop serious or life-threatening problems. Tell your healthcare provider about all medicines you are taking before you begin taking LEXIVA and NORVIR (ritonavir) together.
What should I tell my healthcare provider before taking LEXIVA?
Before taking LEXIVA, tell your healthcare provider about all of your medical conditions including if you:
- are pregnant or planning to become pregnant. It is not known if LEXIVA can harm your unborn baby. You and your healthcare provider will need to decide if LEXIVA is right for you. If you use LEXIVA while you are pregnant, talk to your healthcare provider about how you can be on the Antiretroviral Pregnancy Registry.
- are breastfeeding. You should not breastfeed if you are HIV-positive because of the chance of passing the HIV virus to your baby through your milk. Also, it is not known if LEXIVA can pass into your breast milk and if it can harm your baby. If you are a woman who has or will have a baby, talk with your healthcare provider about the best way to feed your baby.
- have liver problems. You may be given a lower dose of LEXIVA or LEXIVA may not be right for you.
- have kidney problems
- have diabetes. You may need dose changes in your insulin or other diabetes medicines.
- have hemophilia
- are allergic to sulfa medicines
Before taking LEXIVA, tell your healthcare provider about all the medicines you take, including prescription and nonprescription medicines, vitamins, and herbal supplements. LEXIVA can cause dangerous and life-threatening interactions if taken with certain other medicines. You may need dose changes in some of your medicines or closer monitoring with some medicines if you also take LEXIVA (see “What is the most important information I should know about LEXIVA.”). Know all the medicines that you take and keep a list of them with you to show healthcare providers and pharmacists.
Women who use birth control pills should choose a different kind of contraception. The use of LEXIVA with NORVIR (ritonavir) in combination with birth control pills may be harmful to your liver. The use of LEXIVA with or without NORVIR may decrease the effectiveness of birth control pills. Talk to your healthcare provider about choosing an effective contraceptive.
How should I take LEXIVA?
- Take LEXIVA exactly as your healthcare provider prescribed.
- Do not take more or less than your prescribed dose of LEXIVA at any one time. Do not change your dose or stop taking LEXIVA without talking with your healthcare provider.
- You can take LEXIVA Tablets with or without food.
- Adults should take LEXIVA Oral Suspension without food.
- Pediatric patients should take LEXIVA Oral Suspension with food. If vomiting occurs within 30 minutes after dosing, the dose should be repeated.
- Shake LEXIVA Oral Suspension vigorously before each use.
- When your supply of LEXIVA or other anti-HIV medicine starts to run low, get more from your healthcare provider or pharmacy. The amount of HIV virus in your blood may increase if one or more of the medicines are stopped, even for a short time.
- Stay under the care of a healthcare provider while using LEXIVA.
- It is important that you do not miss any doses. If you miss a dose of LEXIVA by more than 4 hours, wait and take the next dose at the regular time. However, if you miss a dose by fewer than 4 hours, take your missed dose right away. Then take your next dose at the regular time.
- If you take too much LEXIVA, call your healthcare provider or poison control center right away.
What should I avoid while taking LEXIVA?
- Do not use certain medicines while you are taking LEXIVA. See “What is the most important information I should know about LEXIVA" and "Who should not take LEXIVA?”
- Do not breastfeed. See “Before taking LEXIVA, tell your healthcare provider”. Talk with your healthcare provider about the best way to feed your baby.
- Avoid doing things that can spread HIV infection since LEXIVA doesn't stop you from passing the HIV infection to others.
- Do not share needles or other injection equipment.
- Do not share personal items that can have blood or body fluids on them, like toothbrushes or razor blades.
- Do not have any kind of sex without protection. Always practice safer sex by using a latex or polyurethane condom to lower the chance of sexual contact with semen, vaginal secretions, or blood.
What are the possible side effects of LEXIVA?
LEXIVA may cause the following side effects:
- skin rash. Skin rashes, some with itching, have happened in patients taking LEXIVA. Swelling of the face, lips, and tongue (angioedema) has also been reported. Tell your healthcare provider if you get a rash or develop facial swelling after starting LEXIVA.
- diabetes and high blood sugar (hyperglycemia). Some patients had diabetes before taking LEXIVA while others did not. Some patients may need changes in their diabetes medicine. Others may need a new diabetes medicine.
- increased bleeding problems in some patients with hemophilia.
- worse liver disease. Patients with liver problems, including hepatitis B or C, are more likely to get worse liver disease when they take anti-HIV medicines like LEXIVA.
- changes in blood tests. Some people have changes in blood tests while taking LEXIVA. These include increases seen in liver function tests and blood fat levels, and decreases in white blood cells. Your healthcare provider may do regular blood tests to see if LEXIVA is affecting your body.
- changes in body fat. These changes have happened in patients taking antiretroviral medicines like LEXIVA. The changes may include an increased amount of fat in the upper back and neck ("buffalo hump"), breast, and around the trunk. Loss of fat from the legs, arms, and face may also happen. The cause and long-term health effects of these conditions are not known at this time.
- kidney stones have been reported in some patients taking LEXIVA. If you develop signs or symptoms of kidney stones (pain in your side, blood in your urine, pain when you urinate) tell your healthcare provider right away.
Common side effects of LEXIVA are nausea, vomiting, and diarrhea. Tell your healthcare provider about any side effects that bother you or that won't go away.
This list of side effects of LEXIVA is not complete. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store LEXIVA?
- LEXIVA Tablets should be stored at room temperature between 59° and 86°F (15° to 30°C). Keep the container of LEXIVA Tablets tightly closed.
- LEXIVA Oral Suspension may be stored at room temperature or refrigerated. Refrigeration of LEXIVA Oral Suspension may improve taste for some patients. Do not freeze.
- Keep LEXIVA and all medicines out of the reach of children.
- Do not keep medicine that is out of date or that you no longer need. Be sure that if you throw any medicine away, it is out of the reach of children.
General information about LEXIVA
Medicines are sometimes prescribed for conditions that are not mentioned in patient information leaflets. Do not use LEXIVA for a condition for which it was not prescribed. Do not give LEXIVA to other people, even if they have the same symptoms you have. It may harm them.
This leaflet summarizes the most important information about LEXIVA. If you would like more information, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about LEXIVA that is written for health professionals. For more information you can call toll-free 888-825-5249 or visit www.LEXIVA.com.
What are the ingredients in LEXIVA?
Tablets:
Active Ingredient: fosamprenavir calcium.
Inactive Ingredients: colloidal silicon dioxide, croscarmellose sodium, magnesium stearate, microcrystalline cellulose, and povidone K30. The tablet film-coating contains the inactive ingredients hypromellose, iron oxide red, titanium dioxide, and triacetin.
LEXIVA Tablets, 700 mg, are pink in color and are capsule-shaped, with the letters “GX LL7” printed on one side of the tablet.
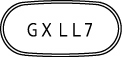
Oral Suspension:
Active Ingredient: fosamprenavir calcium
Inactive ingredients: artificial grape-bubblegum flavor, calcium chloride dihydrate, hypromellose, methylparaben, natural peppermint flavor, polysorbate 80, propylene glycol, propylparaben, purified water, and sucralose.
LEXIVA and AGENERASE are registered trademarks of GlaxoSmithKline.
- The brands listed are trademarks of their respective owners and are not trademarks of GlaxoSmithKline. The makers of these brands are not affiliated with and do not endorse GlaxoSmithKline or its products.
GlaxoSmithKline Vertex Pharmaceuticals Incorporated
Research Triangle Park, NC 27709 Cambridge, MA 02139
©2009, GlaxoSmithKline. All rights reserved.
This Product was Repackaged By:
State of Florida DOH Central Pharmacy
104-2 Hamilton Park Drive
Tallahassee, FL 32304
United States
