Medique at Home Diphen
Medique @ Home Diphen Caplet
b56d1966-efd7-56eb-e053-2995a90a89b5
HUMAN OTC DRUG LABEL
Aug 19, 2025
Unifirst First Aid Corporation
DUNS: 832947092
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Diphenhydramine HCl
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (10)
Drug Labeling Information
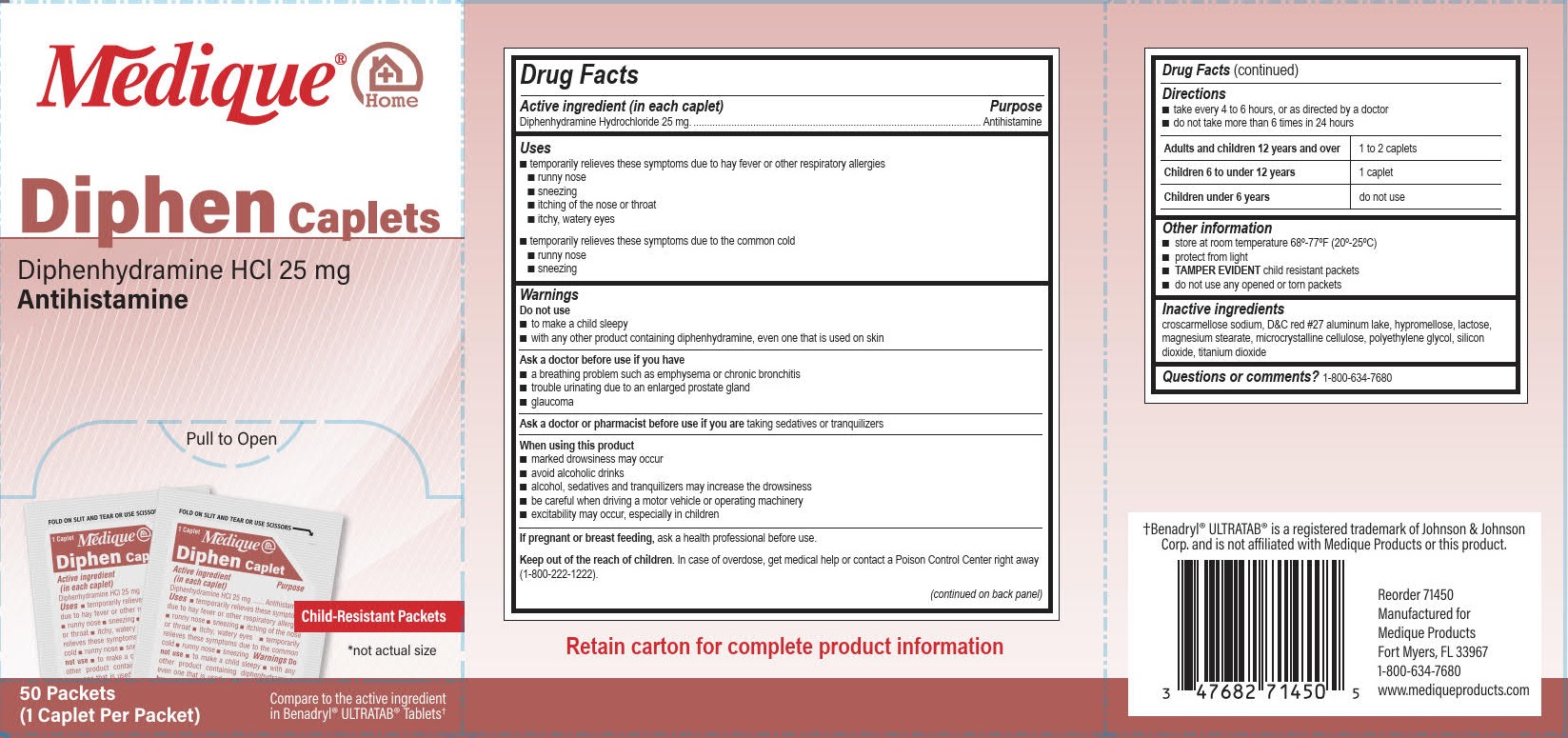
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Medique® @ Home
Diphen Caplets
Diphenhydramine HCl 25 mg
Antihistamine
Pull to Open
Child-Resistant Packets
*not actual size
50 Packets
(1 Caplet Per Packet)
Compare to the active ingredient in Benadryl® Ultratab® Tablets
INDICATIONS & USAGE SECTION
Uses
■ temporarily relieves these symptoms due to hay fever or other respiratory allergies
■ runny nose ■ sneezing ■ itching of the nose or throat ■ itchy, watery eyes
■ temporarily relieves these symptoms due to the common cold
■ runny nose ■ sneezing
SPL UNCLASSIFIED SECTION
Drug Facts
OTC - ACTIVE INGREDIENT SECTION
Active ingredient (in each caplet)
Diphenhydramine Hydrochloride 25 mg
OTC - DO NOT USE SECTION
Do not use
■ to make a child sleepy
■ with any other product containing diphenhydramine, even one that is used on skin.
OTC - WHEN USING SECTION
When using this product
■ marked drowsiness may occur
■ avoid alcoholic drinks
■ alcohol, sedatives and tranquilizers may increase drowsiness
■ be careful when driving a motor vehicle or operating machinery
■ excitability may occur, especially in children
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
Keep out of the reach of children. In case of overdose, get medical help or contact a Poison Control Center right away (1-800-222-1222).
OTHER SAFETY INFORMATION
Other information
■ store at room temperature 68º-77ºF (20º-25ºC)
■ protect from light
■TAMPER EVIDENT child resistant packets
■ do not use any opened or torn packets
OTC - PURPOSE SECTION
Purpose
Antihistamine
OTC - ASK DOCTOR/PHARMACIST SECTION
Ask a doctor or pharmacist before use if you aretaking sedatives or tranquilizers
WARNINGS SECTION
Warnings
OTC - ASK DOCTOR SECTION
Ask a doctor before use if you have
■ a breathing problem such as emphysema or chronic bronchitis
■ trouble urinating due to an enlarged prostate gland
■ glaucoma
OTC - PREGNANCY OR BREAST FEEDING SECTION
**If pregnant or breast feeding,**ask a health professional before use.
DOSAGE & ADMINISTRATION SECTION
Directions
■ take every 4 to 6 hours, or as directed by a doctor
■ do not take more than 6 times in 24 hours
Adults and children12 years and over 1 to 2 caplets
Children 6 to under 12 years 1 caplet
Children under 6 years do not use
INACTIVE INGREDIENT SECTION
Inactive ingredients
croscarmellose sodium, D&C red #27 aluminum lake, hypromellose, lactose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, silicon dioxide, titanium dioxide
OTC - QUESTIONS SECTION
Questions or comments? 1-800-634-7680
