Hydrocortisone and Acetic Acid
Hydrocortisone 1% and Acetic Acid 2% Otic Solution
6383c722-84e2-4719-a13d-19c54e3eefff
HUMAN PRESCRIPTION DRUG LABEL
Jul 7, 2025
Sun Pharmaceutical Industries, Inc.
DUNS: 146974886
Taro Pharmaceuticals U.S.A., Inc.
DUNS: 145186370
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Hydrocortisone and Acetic Acid
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (14)
Drug Labeling Information
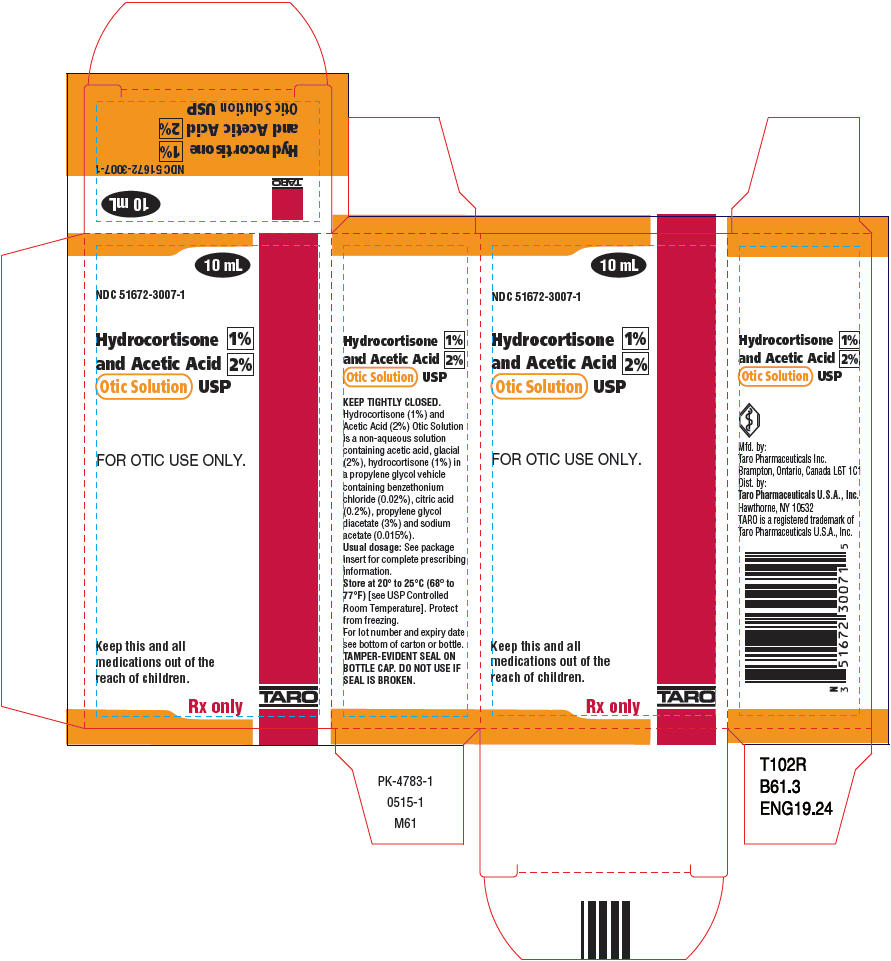
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 10 mL Bottle Carton
10 mL
NDC 51672-3007-1
Hydrocortisone 1%
** and Acetic Acid 2%**
** Otic Solution USP**
FOR OTIC USE ONLY.
Keep this and all
** medications out of the**
** reach of children.**
Rx only
TARO
DESCRIPTION SECTION
DESCRIPTION
Hydrocortisone and Acetic Acid Otic Solution, USP contains Hydrocortisone (1%) and acetic acid, glacial (2%) in a propylene glycol vehicle containing benzethonium chloride (0.02%), citric acid (0.2%), propylene glycol diacetate (3%) and sodium acetate (0.015%).
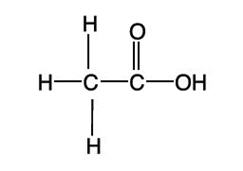
Acetic acid has a molecular formula of CH 3COOH with molecular weight of 60.05. The structural formula is:
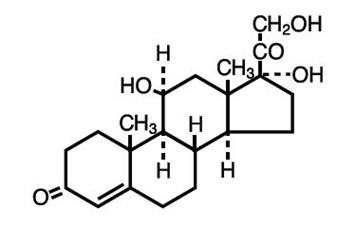
Hydrocortisone is a Synthetic Steroid used as an anti-inflammatory and antipruritic agent. Its chemical name is Pregn-4-ene-3,20-dione, 11, 17, 21-trihydroxy-, (11β)-. Hydrocortisone has a molecular formula of C 21H 30O 5with molecular weight 362.46. The structural formula is:
Hydrocortisone and acetic acid is available as a non-aqueous otic solution buffered at pH (2.0 to 4.0) for use in the external ear canal.
SPL UNCLASSIFIED SECTION
Mfd. by: Taro Pharmaceuticals Inc.
Brampton, Ontario, Canada L6T 1C1
Dist. by:Taro Pharmaceuticals U.S.A., Inc.
Hawthorne, NY 10532
Revised: May, 2015
PK-4785-1
61
0515-1
HOW SUPPLIED SECTION
HOW SUPPLIED
Hydrocortisone 1% and acetic acid 2% otic solution is available in 10 mL plastic, controlled dropper tip bottle.
|
10 mL bottle |
NDC 51672-3007-1 |
Store at 20° to 25°C (68° to 77°F)[see USP Controlled Room Temperature]. Protect from freezing. Keep container tightly closed.
