Nabumetone
Nabumetone Tablets, USPRx only
0186328f-3eb7-4407-bf9e-8007d08ebe7b
HUMAN PRESCRIPTION DRUG LABEL
Sep 23, 2025
Bryant Ranch Prepack
DUNS: 171714327
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Nabumetone
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (11)
Drug Labeling Information
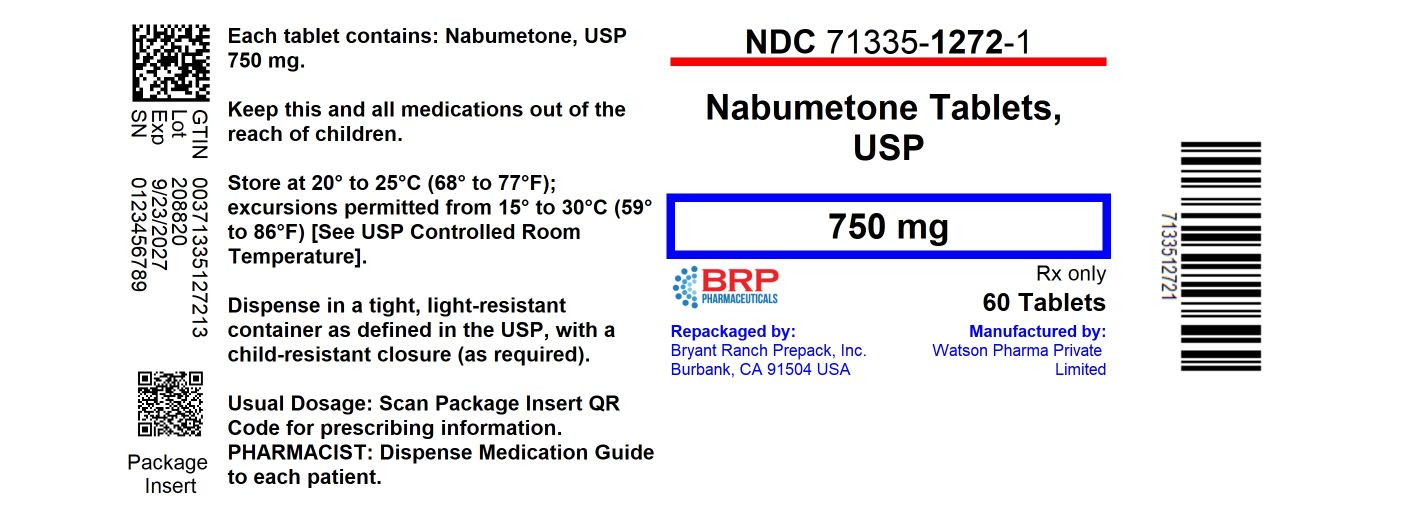
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Nabumetone 750mg Tablets
BOXED WARNING SECTION
Pharmacokinetics
After oral administration, approximately 80% of a radiolabeled dose of nabumetone is found in the urine, indicating that nabumetone is well absorbed from the gastrointestinal tract. Nabumetone itself is not detected in the plasma because, after absorption, it undergoes rapid biotransformation to the principal active metabolite, 6-methoxy-2-naphthylacetic acid (6MNA). Approximately 35% of a 1,000 mg oral dose of nabumetone is converted to 6MNA and 50% is converted into unidentified metabolites which are subsequently excreted in the urine. Following oral administration of nabumetone, 6MNA exhibits pharmacokinetic characteristics that generally follow a one- compartment model with first order input and first order elimination.
6MNA is more than 99% bound to plasma proteins. The free fraction is dependent on total concentration of 6MNA and is proportional to dose over the range of 1,000 mg to 2,000 mg. It is 0.2% to 0.3% at concentrations typically achieved following administration of 1,000 mg of nabumetone and is approximately 0.6% to 0.8% of the total concentrations at steady state following daily administration of 2,000 mg.
Steady-state plasma concentrations of 6MNA are slightly lower than predicted from single-dose data. This may result from the higher fraction of unbound 6MNA which undergoes greater hepatic clearance.
Coadministration of food increases the rate of absorption and subsequent appearance of 6MNA in the plasma but does not affect the extent of conversion of nabumetone into 6MNA. Peak plasma concentrations of 6MNA are increased by approximately one third.
Coadministration with an aluminum-containing antacid had no significant effect on the bioavailability of 6MNA.
Table 1: Mean Pharmacokinetic Parameters of Nabumetone Active Metabolite (6MNA) at Steady State Following Oral Administration of 1,000 mg or 2,000 mg Doses of Nabumetone|
Abbreviation |
Young Adults |
Young Adults |
Elderly |
|
Tmax (hr) |
3.0 (1.0 to 12.0) |
2.5 (1.0 to 8.0) |
4.0 (1.0 to 10.0) |
|
t½ (hr) |
22.5 ± 3.7 |
26.2 ± 3.7 |
29.8 ± 8.1 |
|
CLss/F (mL/min) |
26.1 ± 17.3 |
21.0 ± 4.0 |
18.6 ± 13.4 |
|
Vdss/F (L) |
55.4 ± 26.4 |
53.4 ± 11.3 |
50.2 ± 25.3 |
The simulated curves in the graph below illustrate the range of active metabolite plasma concentrations that would be expected from 95% of patients following 1,000 mg to 2,000 mg doses to steady state. The cross-hatched area represents the expected overlap in plasma concentrations due to intersubject variation following oral administration of 1,000 mg to 2,000 mg of nabumetone.

Nabumetone Active Metabolite (6MNA) Plasma Concentrations at Steady State Following Once-Daily Dosing of Nabumetone 1,000 mg (n = 31) 2,000 mg (n = 12)
6MNA undergoes biotransformation in the liver, producing inactive metabolites that are eliminated as both free metabolites and conjugates. None of the known metabolites of 6MNA has been detected in plasma. Preliminary in vivo and in vitro studies suggest that unlike other NSAIDs, there is no evidence of enterohepatic recirculation of the active metabolite. Approximately 75% of a radiolabeled dose was recovered in urine in 48 hours. Approximately 80% was recovered in 168 hours. A further 9% appeared in the feces. In the first 48 hours, metabolites consisted of:
|
–nabumetone, unchanged |
not detectable |
|
–6-methoxy-2-naphthylacetic acid |
<1% |
|
–6MNA, conjugated |
11% |
|
–6-hydroxy-2-naphthylacetic acid |
5% |
|
–6HNA, conjugated |
7% |
|
–4-(6-hydroxy-2-naphthyl)-butan-2-ol, Conjugated |
9% |
|
–O-desmethyl-nabumetone, conjugated |
7% |
|
–unidentified minor metabolites |
34% |
|
Total % Dose: |
73% |
Following oral administration of dosages of 1,000 mg to 2,000 mg to steady state, the mean plasma clearance of 6MNA is 20 to 30 mL/min and the elimination half-life is approximately 24 hours.
Elderly Patients
Steady-state plasma concentrations in elderly patients were generally higher than in young healthy subjects (seeTable 1 for summary of pharmacokinetic parameters).
Renal Insufficiency
In moderate renal insufficiency patients (creatinine clearance 30 to 49 mL/min), the terminal half-life of 6MNA was increased by approximately 50% (39.2 ± 7.8 hrs, N = 12) compared to the normal subjects (26.9 ± 3.3 hrs, N = 13), and there was a 50% increase in the plasma levels of unbound 6MNA.
Additionally, the renal excretion of 6MNA in the moderate renal impaired patients decreased on average by 33% compared to that in the normal patients. A similar increase in the mean terminal half-life of 6MNA was seen in a small study of patients with severe renal dysfunction (creatinine clearance < 30 mL/min). In patients undergoing hemodialysis, steady-state plasma concentrations of the active metabolite 6MNA were similar to those observed in healthy subjects. Due to extensive protein binding, 6MNA is not dialyzable.
Dosage adjustment of nabumetone generally is not necessary in patients with mild renal insufficiency (≥50 mL/min). Caution should be used in prescribing nabumetone to patients with moderate or severe renal insufficiency. The maximum starting doses of nabumetone in patients with moderate or severe renal insufficiency should not exceed 750 mg or 500 mg, respectively once daily. Following careful monitoring of renal function in patients with moderate or severe renal insufficiency, daily doses may be increased to a maximum of 1,500 mg and 1,000 mg, respectively (seeWARNINGS,** Renal Effects**).
Hepatic Impairment
Data in patients with severe hepatic impairment are limited. Biotransformation of nabumetone to 6MNA and the further metabolism of 6MNA to inactive metabolites is dependent on hepatic function and could be reduced in patients with severe hepatic impairment (history of or biopsy-proven cirrhosis).
CLINICAL PHARMACOLOGY SECTION
CLINICAL PHARMACOLOGY
Nabumetone is a non-steroidal anti-inflammatory drug (NSAID) that exhibits anti-inflammatory, analgesic, and antipyretic properties in pharmacologic studies. As with other non-steroidal anti-inflammatory agents, its mode of action is not known; however, the ability to inhibit prostaglandin synthesis may be involved in the anti-inflammatory effect.
The parent compound is a prodrug, which undergoes hepatic biotransformation to the active component, 6-methoxy-2-naphthylacetic acid (6MNA), that is a potent inhibitor of prostaglandin synthesis.
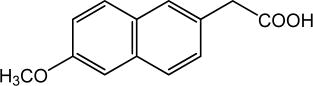
6-methoxy-2-naphthylacetic acid (6MNA)
It is acidic and has an n-octanol: phosphate buffer partition coefficient of 0.5 at pH 7.4.
Pharmacokinetics
After oral administration, approximately 80% of a radiolabeled dose of nabumetone is found in the urine, indicating that nabumetone is well absorbed from the gastrointestinal tract. Nabumetone itself is not detected in the plasma because, after absorption, it undergoes rapid biotransformation to the principal active metabolite, 6-methoxy-2-naphthylacetic acid (6MNA). Approximately 35% of a 1,000 mg oral dose of nabumetone is converted to 6MNA and 50% is converted into unidentified metabolites which are subsequently excreted in the urine. Following oral administration of nabumetone, 6MNA exhibits pharmacokinetic characteristics that generally follow a one- compartment model with first order input and first order elimination.
6MNA is more than 99% bound to plasma proteins. The free fraction is dependent on total concentration of 6MNA and is proportional to dose over the range of 1,000 mg to 2,000 mg. It is 0.2% to 0.3% at concentrations typically achieved following administration of 1,000 mg of nabumetone and is approximately 0.6% to 0.8% of the total concentrations at steady state following daily administration of 2,000 mg.
Steady-state plasma concentrations of 6MNA are slightly lower than predicted from single-dose data. This may result from the higher fraction of unbound 6MNA which undergoes greater hepatic clearance.
Coadministration of food increases the rate of absorption and subsequent appearance of 6MNA in the plasma but does not affect the extent of conversion of nabumetone into 6MNA. Peak plasma concentrations of 6MNA are increased by approximately one third.
Coadministration with an aluminum-containing antacid had no significant effect on the bioavailability of 6MNA.
Table 1: Mean Pharmacokinetic Parameters of Nabumetone Active Metabolite (6MNA) at Steady State Following Oral Administration of 1,000 mg or 2,000 mg Doses of Nabumetone|
Abbreviation |
Young Adults |
Young Adults |
Elderly |
|
Tmax (hr) |
3.0 (1.0 to 12.0) |
2.5 (1.0 to 8.0) |
4.0 (1.0 to 10.0) |
|
t½ (hr) |
22.5 ± 3.7 |
26.2 ± 3.7 |
29.8 ± 8.1 |
|
CLss/F (mL/min) |
26.1 ± 17.3 |
21.0 ± 4.0 |
18.6 ± 13.4 |
|
Vdss/F (L) |
55.4 ± 26.4 |
53.4 ± 11.3 |
50.2 ± 25.3 |
The simulated curves in the graph below illustrate the range of active metabolite plasma concentrations that would be expected from 95% of patients following 1,000 mg to 2,000 mg doses to steady state. The cross-hatched area represents the expected overlap in plasma concentrations due to intersubject variation following oral administration of 1,000 mg to 2,000 mg of nabumetone.
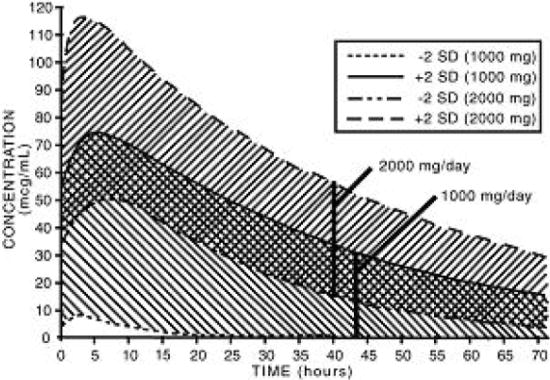
Nabumetone Active Metabolite (6MNA) Plasma Concentrations at Steady State Following Once-Daily Dosing of Nabumetone 1,000 mg (n = 31) 2,000 mg (n = 12)
6MNA undergoes biotransformation in the liver, producing inactive metabolites that are eliminated as both free metabolites and conjugates. None of the known metabolites of 6MNA has been detected in plasma. Preliminary in vivo and in vitro studies suggest that unlike other NSAIDs, there is no evidence of enterohepatic recirculation of the active metabolite. Approximately 75% of a radiolabeled dose was recovered in urine in 48 hours. Approximately 80% was recovered in 168 hours. A further 9% appeared in the feces. In the first 48 hours, metabolites consisted of:
|
–nabumetone, unchanged |
not detectable |
|
–6-methoxy-2-naphthylacetic acid |
<1% |
|
–6MNA, conjugated |
11% |
|
–6-hydroxy-2-naphthylacetic acid |
5% |
|
–6HNA, conjugated |
7% |
|
–4-(6-hydroxy-2-naphthyl)-butan-2-ol, Conjugated |
9% |
|
–O-desmethyl-nabumetone, conjugated |
7% |
|
–unidentified minor metabolites |
34% |
|
Total % Dose: |
73% |
Following oral administration of dosages of 1,000 mg to 2,000 mg to steady state, the mean plasma clearance of 6MNA is 20 to 30 mL/min and the elimination half-life is approximately 24 hours.
Elderly Patients
Steady-state plasma concentrations in elderly patients were generally higher than in young healthy subjects (seeTable 1 for summary of pharmacokinetic parameters).
Renal Insufficiency
In moderate renal insufficiency patients (creatinine clearance 30 to 49 mL/min), the terminal half-life of 6MNA was increased by approximately 50% (39.2 ± 7.8 hrs, N = 12) compared to the normal subjects (26.9 ± 3.3 hrs, N = 13), and there was a 50% increase in the plasma levels of unbound 6MNA.
Additionally, the renal excretion of 6MNA in the moderate renal impaired patients decreased on average by 33% compared to that in the normal patients. A similar increase in the mean terminal half-life of 6MNA was seen in a small study of patients with severe renal dysfunction (creatinine clearance < 30 mL/min). In patients undergoing hemodialysis, steady-state plasma concentrations of the active metabolite 6MNA were similar to those observed in healthy subjects. Due to extensive protein binding, 6MNA is not dialyzable.
Dosage adjustment of nabumetone generally is not necessary in patients with mild renal insufficiency (≥50 mL/min). Caution should be used in prescribing nabumetone to patients with moderate or severe renal insufficiency. The maximum starting doses of nabumetone in patients with moderate or severe renal insufficiency should not exceed 750 mg or 500 mg, respectively once daily. Following careful monitoring of renal function in patients with moderate or severe renal insufficiency, daily doses may be increased to a maximum of 1,500 mg and 1,000 mg, respectively (seeWARNINGS,** Renal Effects**).
Hepatic Impairment
Data in patients with severe hepatic impairment are limited. Biotransformation of nabumetone to 6MNA and the further metabolism of 6MNA to inactive metabolites is dependent on hepatic function and could be reduced in patients with severe hepatic impairment (history of or biopsy-proven cirrhosis).
Special Studies
Gastrointestinal
Nabumetone was compared to aspirin in inducing gastrointestinal blood loss. Food intake was not monitored. Studies utilizing 51Cr-tagged red blood cells in healthy males showed no difference in fecal blood loss after 3 or 4 weeks' administration of 1,000 mg or 2,000 mg of nabumetone daily when compared to either placebo-treated or non-treated subjects. In contrast, aspirin 3,600 mg daily produced an increase in fecal blood loss when compared to subjects who received nabumetone, placebo, or no treatment. The clinical relevance of the data is unknown.
The following endoscopy trials entered patients who had been previously treated with NSAIDs. These patients had varying baseline scores and different courses of treatment. The trials were not designed to correlate symptoms and endoscopy scores. The clinical relevance of these endoscopy trials, i.e., either G.I. symptoms or serious G.I. events, is not known.
Ten endoscopy studies were conducted in 488 patients who had baseline and post-treatment endoscopy. In 5 clinical trials that compared a total of 194 patients on 1,000 mg of nabumetone daily or naproxen 250 mg or 500 mg twice daily for 3 to 12 weeks, treatment with nabumetone resulted in fewer patients with endoscopically detected lesions (> 3 mm). In 2 trials a total of 101 patients administered 1,000 mg or 2,000 mg of nabumetone daily or piroxicam 10 mg to 20 mg for 7 to 10 days, there were fewer patients treated with nabumetone with endoscopically detected lesions. In 3 trials of a total of 47 patients on 1,000 mg of nabumetone daily or indomethacin 100 mg to 150 mg daily for 3 to 4 weeks, the endoscopy scores were higher with indomethacin. Another 12 week trial in a total of 171 patients compared the results of treatment with 1,000 mg of nabumetone daily to ibuprofen 2,400 mg/day and ibuprofen 2,400 mg/day plus misoprostol 800 mcg/day. The results showed that patients treated with nabumetone had a lower number of endoscopically detected lesions (> 5 mm) than patients treated with ibuprofen alone but comparable to the combination of ibuprofen plus misoprostol. The results did not correlate with abdominal pain.
Other
In 1 week, repeat-dose studies in healthy volunteers, 1,000 mg of nabumetone daily had little effect on collagen-induced platelet aggregation and no effect on bleeding time. In comparison, naproxen 500 mg daily suppressed collagen- induced platelet aggregation and significantly increased bleeding time.
DESCRIPTION SECTION
DESCRIPTION
Nabumetone, USP is a naphthylalkanone designated chemically as 4-(6-methoxy-2-naphthalenyl)-2-butanone. It has the following structure:
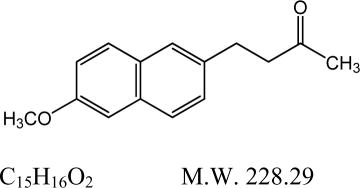
Nabumetone, USP is a white to off-white crystalline substance. It is nonacidic and practically insoluble in water, but soluble in alcohol and most organic solvents. It has an n-octanol: phosphate buffer partition coefficient of 2,400 at pH 7.4.
Each tablet, for oral administration, contains either 500 mg or 750 mg of nabumetone, USP. In addition, each tablet contains the following inactive ingredients: colloidal silicon dioxide, corn starch, hypromellose, magnesium stearate, povidone, sodium lauryl sulfate, sodium starch glycolate, titanium dioxide and triacetin. The 500 mg tablets also contain talc and the 750 mg tablets also contain iron oxide red.
HOW SUPPLIED SECTION
HOW SUPPLIED
Nabumetone tablets USP, 750 mg are pink, oval-shaped, film-coated, biconvex tablets debossed with “3671” on one side of the tablet and plain on the other side. They are available in
- NDC 71335-1272-1: 60 Tablets in a BOTTLE
- NDC 71335-1272-2: 90 Tablets in a BOTTLE
- NDC 71335-1272-3: 30 Tablets in a BOTTLE
- NDC 71335-1272-4: 20 Tablets in a BOTTLE
- NDC 71335-1272-5: 42 Tablets in a BOTTLE
- NDC 71335-1272-6: 100 Tablets in a BOTTLE
- NDC 71335-1272-7: 14 Tablets in a BOTTLE
- NDC 71335-1272-8: 6 Tablets in a BOTTLE
- NDC 71335-1272-9: 9 Tablets in a BOTTLE
- NDC 71335-1272-0: 7 Tablets in a BOTTLE
Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature].
Dispense in a tight, light-resistant container as defined in the USP, with a child-resistant closure (as required).
Repackaged/Relabeled by:
Bryant Ranch Prepack, Inc.
Burbank, CA 91504
