Vancomycin Hydrochloride
Vancomycin Hydrochloride
0713a847-a334-4b73-af22-343aa06621b9
HUMAN PRESCRIPTION DRUG LABEL
Feb 7, 2023
Fresenius Kabi USA, LLC
DUNS: 608775388
Products 2
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
VANCOMYCIN HYDROCHLORIDE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (1)
VANCOMYCIN HYDROCHLORIDE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (1)
Drug Labeling Information
DESCRIPTION SECTION
DESCRIPTION
Vancomycin Hydrochloride for Injection, USP, is an off-white to buff-colored lyophilized powder, for preparing intravenous (IV) infusions, in Pharmacy Bulk Package bottles containing the equivalent of 5 g or 10 g vancomycin base. 500 mg of the base are equivalent to 0.34 mmol.
When reconstituted with Sterile Water for Injection to a concentration of 50 mg/mL for the 5 g Pharmacy Bulk Package bottle and 100 mg/mL for the
10 g Pharmacy Bulk Package bottle, the pH of the solution is between 2.5 and 4.5. This product is oxygen sensitive. Vancomycin Hydrochloride for Injection, USP should be administered intravenously in diluted solution (seeDOSAGE AND ADMINISTRATION),AFTER RECONSTITUTION FURTHER DILUTION IS REQUIRED BEFORE USE.
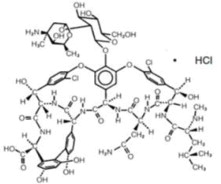
Vancomycin is a tricyclic glycopeptide antibiotic derived from Amycolatopasis orientalis (formerly Nocardia orientalis). The chemical name for vancomycin hydrochloride is 3S-[3R*,6S*(S*),7S*,22S*,23R*,26R*,36S*,38aS*]]-3-(2-Amino-2-oxoethyl)-44-[[2-O-(3-amino- 2,3,6-trideoxy-3-C-methyl-α-L-lyxo-hexopyranosyl)-ß-D- glucopyranosyl]oxy]-10,19-dichloro- 2,3,4,5,6,7,23,24,25,26,36,37,38,38a-tetradecahydro-7,22,28,30,32-pentahydroxy-6-[[4-methyl- 2-(methylamino)-1-oxopentyl]amino]-2,5,24,38,39-pentaoxo-22H-8,11:18,21-dietheno-23,36-(iminomethano)-13,16:31,35-dimetheno-1H,16H-[1,6,9]oxadiazacyclohexadecino[4,5- m][10,2,16]-benzoxadiazacyclotetracosine-26-carboxylic acid, monohydrochloride. The molecular formula is C66H75Cl2N9O24• HCl and the molecular weight is 1,485.74. Vancomycin hydrochloride has the following structural formula:
A pharmacy bulk package is a container of a sterile preparation for parenteral use that contains many single doses. The contents of this pharmacy bulk
package are intended for use by a pharmacy admixture service for addition to suitable parenteral fluids in the preparation of admixtures for intravenous infusion (SeeDOSAGE AND ADMINISTRATION, Directions for Proper Use of Pharmacy Bulk Package) FURTHER DILUTION IS REQUIRED. NOT FOR DIRECT INFUSION.
REFERENCES SECTION
REFERENCES
- Moellering RC, Krogstad DJ, Greenblatt DJ: Vancomycin therapy in patients with impaired renal function: A nomogram for dosage. Ann Inter Med 1981; 94:343.
The brand names mentioned in this document are the trademarks of their respective owners/companies.
PREMIERProRx® is a registered trademark of Premier Healthcare Alliance, L.P., used under license.
Manufactured by:
Fresenius Kabi
****Lake Zurich, IL 60047
www.fresenius-kabi.com/us
451447C
Revised: December 2021

