TESTO-200
Testo-200 inj
09ffa1bb-23f9-a399-e063-6294a90a7ccd
HUMAN PRESCRIPTION DRUG LABEL
Feb 21, 2024
Advanced Pharmaceutical Technology, Inc.
DUNS: 023237884
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
testosterone cypionate inj.
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (3)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
TESTO-200 (57377-200-01)
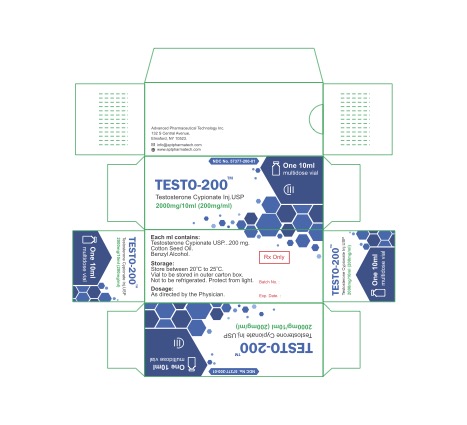
Caution:
Federal Law prohibits dispensing without presciption.
Store at room temperature.
INDICATIONS & USAGE SECTION
INDICATIONS AND USAGE
Testosterone Cypionate Injection, USP is indicated for replacement therapy in tho male in conditions associated with symptoms of deficiency or absence of endogenous testosterone.
1. Primary hypogonadism (congenital or acquired): testicular failure due to
cryptorchidism, bilateral torsion, orchitis, vanishing testis syndrome; or
orchidectorny.
2. Hypogonadotropic hypogonadism (congenital or acquired): gonadotropin or
luteinizing hormone-releasing hormone (LHRH) deficiency, or pituitary-
hypothalamic injury from tumors, trauma, or radiation.
CONTRAINDICATIONS SECTION
CONTRAINDICATIONS
1. Known hypersensitivity to lhe drug
2. Males with carcinoma of the breast
3. Males with known or suspected carcinoma of the prostate gland
4. Women who are pregnant (see PRECUTIONS, Pregnancy)
5. Palients with serious cardiac, hepatic or renal disease
(see WARNINGS)
ADVERSE REACTIONS SECTION
ADVERSE REACTIONS
The following adverse reactions in the male have occurred with some androgens:
Endocrine and urogenital: Gynecomastia and excessive frequency and
duration or penile erections. Oligospermia may occur at high dosages. Skin and
appendages: Hirsutism, male pattern of baldness, seborrhea, and acne.
Cardiovascular Disorders: Myocardial infarction, stroke
Fluid and electrolyte disturbances: Retention of sodium, chloride, water,
potassium, calcium, and inorganic phosphates.Gastrointestinal: Nausea,
cholestalic jaundice, alterations in liver funclion tests, rarely
hepatocellular neoplasms and peliosis hepatis
(see WARNINGS).
Hematologic: Suppression of clotting factors II, V, VII, and X, bleeding
in patients on concomitant anticoagulant therapy, and polycythemia.
Nervous system: Increased or decreased libido, headache, anxiety,
depression, and generalized paresthesia.
Allergic: Hypersensitivity, including skin manifestations and
anaphylactoid reactions.
Vascular Disorders: Venous thromboembolism.
Special senses: Rare cases of central serous chorioretinopathy (CSCR).
Miscellaneous: lnflammafion and pain at the site or intramuscular
injection.
To report SUSPECTED ADVERSE REACTIONS, contact your doctor or physician or FDA
at 1-800-FDA-1088 or www.fda.gov/medwatch.
OVERDOSAGE SECTION
OVERDOSAGE
There have been no reports of acute overdosage with the androgens.
CLINICAL PHARMACOLOGY SECTION
CLINICAL PHARMACOLOGY
Endogenous androgens are responsible for normal growth and development of the
male sex organs and for maintenance of secondary sex characteristics. These
effects include growth and maturation of the prostate, seminal vesicles,
penis, and scrotum; development of male hair distribution, such as beard,
pubic, chest, and axillary hair; laryngeal enlargement, vocal cord thickening,
and alterations in body musculature and fat distribution. Drugs in this class
also cause retention of nitrogen, sodium, potassium, and phosphorus, and
decreased urinary excretion of calcium. Androgens have been reported to
increase protein anabolism and decrease protein catabolism. Nitrogen balance
is improved only when there is sufficienl intake of calories and prolein.
Androgens are responsible for the growth spurt of adolescence and for eventual
termination of linear growth, brought about by fusion of the epiphyseal growth
centers. In children, exogenous androgens accelerate linear growth rates, but
may cause disproportionate advancement in bone maturation. Use over long
periods may result in fusion of the epiphyseal growlh centers and termination
of the growth process. Androgens have been reported to stimulate production of
red blood cells by enhancing production of erythropoietic stimulation factor.
During oxogonous administration of androgens, endogenous tostostorono release
is inhibited through feedback inhibition of pituitary luteinizing hormone (I
H). At large doses of exogenous androgens, spermatogenesis may also be
suppressed through feedback inhibition of pituitary follicle stimulating
hormone (FSH).
There is a lack of substantial evidence that androgens are effective in
fractures, surgery, convalescence, and functional uterine bleeding.
Pharmacokinetics
Testosterone esters are less polar than free testosterone. Testosterone esters
in oil injected intramuscularly are absorbed slowly from the lipid phase;
thus, testosterone cypionate can be given at intervals or two to four weeks.
testosterone in plasma is 98 percent bound to a specific
testosteroneestradiol binding globulin, and about 2 percent is free.
Generally, the amount of this sex-hormone binding globulin in the plasma will
determine the distribution of testosterone between free and bound forms, and
the free testoslerone concentration will determine its half-lire.
About 90 percent of a dose of testosterone is excreted in the urine as
glucuronic and sulfuric acid conjugates of testosterone and its metabolites;
about 6 percent of a dose is oxcrotod in tho locos, mostly in tho unconjugated
form. Inactivation of testosterone occurs primarily in the liver. Testosterone
is metaboli7ed to various 17-keto steroids through two different pathways.
The half-life of testosterone cypionate when injected intramuscularly is
approximately eight days.
In many tissues the activity of testosterone appears to depend on reduction to
dihydrotestosterone, which binds to cytosol receptor proteins. The steroid-
receptor complex is transported to the nucleus where it initiates
transcriplion events and cellular changes relaled to androgen action.
WARNINGS SECTION
WARNINGS
Hyporcalcomia may occur in immobilized patients. If this occurs, tho drug
should be discontinued.
Prolonged usa of high doses of androgens (principally tha 17-a
alkylandrogens) has been associated with development of hapalic adenomas,
hepatocellular carcinoma, and peliosis hepatis - all potentially life
threatening complications.
Geriatric patients treated with androgens may be at an increasod risk of
developing prostatic hypertrophy and prostatic carcinoma although conclusive
evidence to support this concept is lacking.
Thara have been postmarketing reports of venous thromboembolic events,
including deep vein thrombosis (DVT) and pulmonary embolism (PC), in patients
using testosterone products, such as testosterone cypionate. Evaluate patients
who report symptoms of pain, edema, warmth and erythema in the lower extremity
for DVT and those who present with acute shortness of breath for PE. If a
venous thromboembolic event is suspected, discontinue treatment with
testosterone cypionate and initiate appropriate workup and management.
Long term clinical safety trials have not been conducted to assess tha
cardiovascular outcomes of testosterone replacement therapy in men. To date,
apidemiologic studies and randomized controlled trials have been inconclusive
for determining the risk of major adverse cardiovascular events (MACE), such
as non-fatal myocardial infarction, non-fatal stroke, and cardiovascular
daalh, wilh lhe use or testosterone compared to nonuse. Some studies, but not
all, have reported an increased risk of MACE in association with use of
testosterone replacement therapy in men. Patients should be informed of this
possible risk when deciding whether to use or to continue to use testosterone
cypionate.
Testosterone has been subject to abuse, typically at doses higher than
recommended for Iha approved indication and in combination with other anabolic
androgenic steroids. Anabolic androgenic steroid abuse can lead to serious
cardiovascular and psychiatric adverse reactions (see DRUG ABUSE AND
DEPENDENCE).
If testosterone abuse is suspected, check serum testosterone concentrations to
ensure they are within therapeutic range. However, testosterone levels may be
in the normal or subnormal range in man abusing synlhalic teslosterone
derivatives. Counsel patianls concerning lhe serious adverse reactions
associated with abuse of testosterone and anabolic androgenic steroids.
Conversely, consider the possibility of tostosterono and anabolic androgenic
steroid abuse in suspoctod patients who present with serious cardiovascular or
psychiatric adverse events.
Edema, with or without congestive heart failure, may be a serious complication
in patients with pre-existing cardiac, renal or hepalic disease.
Gynecomastia may develop and occasionally persists in patients being treated
for hypogonadism.
The preservative benzyl alcohol has been associated with serious adverse
events, including the "gasping syndrome", and death in pediatric patients.
Although normal therapeutic doses of this product ordinarily deliver amounts
or benzyl alcohol thal era substantially lower than those reported in
association with the "gasping syndrome", the minimum amount of benzyl alcohol
at which toxicity may occur is not known. The risk of benzyl alcohol toxicity
depends on the quantity administered and the liver and kidneys' capacity to
detoxify the chemical. Premature and low-birth weight infants may be more
likely to develop toxicity.
Androgen therapy should be used cautiously in healthy males with delayed
puberty. The effect on bone maturation should be monitored by assessing bone
age of the wrist and hand every 6 months. In children, androgen treatment may
accelerate bone maturation without producing compensatory gain in linear
growth. This adverse effect may result in compromised adult stature. The
younger the child the greater the risk of compromising final mature height.
This drug has not been shown to be safe and effective for the enhancement of
athletic performance. Because of the potential risk of serious adverse health
effects, this drug should not be used for such purpose.
PRECAUTIONS SECTION
PRECAUTIONS
General
Patients with benign prostatic hypertrophy may davalop acute urethral
obstruction. Priapism or excessive sexual stimulation may develop.
Oligospermia may occur after prolonged administration or excessive dosage. If
any of these effects appear, the androgen should be stopped and if restarted,
a lower dosage should be utilized.
Testosterone cypionate should not be used interchangeably with testosterone
propionate because of differences in duration or action. Testosterone
cypionate is not for intravenous use.
Information for patients
Patients should be instructed to report any of the following: nausea, vomiting, changes in skin color, ankle swelling, too frequent or persistent erections of the penis.
Laboratory tests
Hemoglobin and hematocrit levels (to detect polycythemia) should be checked
periodically in patients receiving long-term androgen administration.
Serum cholesterol may increase during androgen therapy.
Drug interactions
Androgens may increase sensitivity to oral anticoagulants. Dosage of the
anticoagulant may require reduction in order to maintain satisfactory
therapeutic hypoprothrombinemia.
Concurrent administration of oxyphenbutazone and androgens may result in
elevated serum levels of oxyphenbutazone.
In diabetic patients, the metabolic effects of androgens may decrease blood
glucose and, therefore, insulin requirements.
Drug/Laboratory test Interferences
Androgens may decrease levels or thyroxine-binding globulin, resulling in decreased total T4 serum levels and increased resin uptake of T3 and T4. Free thyroid hormone levels remain unchanged, however, and there is no clinical evidence of thyroid dysfunction.
Carcinogenesis
Animal data
Testosterone has been tested by subcutaneous injection and implantation in mice and rals. The implant induced cervical-ulerine tumors in mice, which metastasized in some cases. There is suggestive evidence that injection of testosterone into some strains of female mice increases their susceptibility to hepatoma. Testosterone is also known to increase the number of tumors and decrease the degree of differentiation of chemically induced carcinomas of the liver in rats.
Human data
There are rare reports of hepatocellular carcinoma in patients receiving long-
term therapy with androgens in high doses. Withdrawal of the drugs did not
load to regression of the tumors in all cases.
Geriatric patients treated with androgens may be at an increased risk of
developing prostatic hypertrophy and prostatic carcinoma although conclusive
evidence to support this concept is lacking.
Pregnancy
Teratogenic Effects
The use of testosterone in women who are pregnant is contraindicated. Testosterone is teratogenic and may cause fetal harm. Testosterone is known to cause virilization of the female fetus when administrated to pregnant women. Benzyl alcohol can cross the placenta. See WARNINGS.
Nursing mothers
Testosterone cypionate injection is not recommended for use in nursing mothers.
Pediatric use
Safety and effectiveness in pedialric patients below the age of 12 years have not been established
DRUG ABUSE AND DEPENDENCE SECTION
DRUG ABUSE AND DEPENDENCE
Controlled Substance
Testosterone Cypionate Injection contains testosterone, a Schedule Ill controlled substance in the Controlled Substances Act.
Abuse
Drug abuse is intentional non-therapeutic use of a drug, even once, for its rewarding psychological and physiological effects. Abuse and misuse of testosterone are seen in male and female adults and adolescents. Testosterone, often in combination with other anabolic androgenic steroids (AAS), and not obtained by prescription through a pharmacy, may be abused by athletes and bodybuilders. There have been reports of misuse by men taking higher doses of legally obtained testosterone than prescribed and continuing testosterone despite adverse events or against medical advice.
Abuse-Related Adverse Reactions
Serious adverse reactions have been reported in individuals who abuse anabolic
androgenic steroids and include cardiac arrest. myocardial infarction,
hypertrophic cardiomyopathy, congestive heart failure, cerebrovascular
accident, hepatotoxicity, and serious psychiatric manifestations, including
major depression, mania, paranoia, psychosis, delusions, hallucinations,
hostility and aggression.
The following adverse reactions have also been reported in men: transient
ischemic attacks, convulsions, hypomania, irritability, dyslipidemias,
testicular atrophy, subfertility, and infertility.
The following additional adverse reactions have been reported in women:
hirsutism, virilization, deepening of voice, clitoral enlargement, breast
atrophy, male-pattern baldness, and menstrual irregularities.
The following adverse reactions have been reported in male and female
adolescents: premature closure of bony epiphyses with termination of growth,
and precocious puberty.
Because these reactions are reported voluntarily from a population of
uncertain size and may include abuse of other agents, it is not always
possible to reliably estimate their frequency or establish a causal
relationship to drug exposure.
Dependence
Behaviors Associated with AddictionContinued abuse of testosterone and other anabolic steroids, leading to addiction is characterized by the following behaviors:
- Taking greater dosages than prescribed
- Continued drug use despite medical and social problems due to drug use
- Spending significant time to obtain the drug when supplies of the drug are interrupted
- Giving a higher priority to drug use than other obligations
- Having difficulty in discontinuing the drug despite desires and attempts to do so
- Experiencing withdrawal symptoms upon abrupt discontinuation of use
Physical dependence is characterized by withdrawal symptoms after abrupt drug
discontinuation or a significant dose reduction of a drug. Individuals taking
supratherapeutic doses of testosterone may experience withdrawal symptoms
lasting for weeks or months which include depressed mood, major depression,
fatigue, craving, restlessness, irritability, anorexia, insomnia, decreased
libido and hypogonadotropic hypogonadism.
Drug dependence in individuals using approved doses of testosterone for
approved indications has not been documented.
DESCRIPTION SECTION
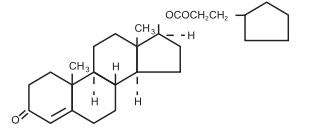
DESCRIPTION
Testosterone Cypionate Injection, USP for intramuscular injection, contains
Testosterone Cypionate, USP which is the oil-soluble 17
(beta)cyclopentylpropionate ester of the androgenic hormone testosterone.
Testosterone Cypionate, USP is a white or creamy white crystalline powder,
odorless or nearly so and stablo in air. It is insoluble in water, freely
soluble in alcohol, chloroform, dioxane, ether, and soluble in vegetable oils.
The chemical name for Testosterone Cypionate, USP is androst-4-en-3- one, 17
-(3-cyclopentyl-1-oxopropoxy)-, ( 17fl )-. Its molecular formula is C27H40O3,
and the rnolecular weight 412.61.
The structural formula is represented below:
Testosterone Cypionate Injection, USP is available in one strength, 200 mg/ml
Testosterone Cypionate, USP.
Each rnl of the 200 rng/rnl solulion conlains:
Testosterone Cypionate 200 mg , USP Benzyl Benzoate 0.2 mL, USP
Bonzyl Alcohol, USP (as preservative) 9.45 mg
DOSAGE & ADMINISTRATION SECTION
DOSAGE AND ADMINISTRATION
Prior to initiating testosterone cypionate, confirm the diagnosis of
hypogonadism by ensuring that serum testosterone concentrations have been
measured in the morning on at least two separate days and that lhese serum
testoslerone concentrations are below lhe normal range.
Testosterone cypionate injection is for intramuscular use only.
It should not bo given intravonously. Intramuscular injections should bo given
deep in the gluteal muscle.
The suggested dosage for testosterone cypionate injection varies depending on
the age, sex, and diagnosis of the individual patient Dosage is adjusted
according to the patient's response and the appearance of adverse reactions.
Various dosage regimens have been used to induce pubertal changes in
hypogonadal males; some experts have advocated lower dosages initially,
gradually increasing the dose as puberty progresses, with or without a
decrease to maintenance levels. Other experts emphasize that higher dosages
are needed lo induce pubertal changes and lower dosages can be used for
maintenance after puberty. The chronological and skeletal ages must be taken
into consideration, both in determining the initial dose and in adjusting the
dose.
For replacement in the hypogonadal male, 50 to 400 mg should be administered
every two to four weeks.
Parenteral drug producls should be inspected visually for particulate rnatter
and discoloration prior to administration, whenever solution and container
permit. Warming and shaking the vial should redissolve any crystals that may
have formed during storage at temperatures lower than recommended. Crystals
does not usually occur when stored properly. Presentation:
Testosterone Cypionate lnjeclion. USP, 200 mg/ml is a clear, pale yellow
oleaginous viscous, sterile solution intended for intramuscular administration
available as:
10 ml Vial, 2000mgl 10ml, 200mg/ml
Storage and handling
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
Protect from lighl.
SPL UNCLASSIFIED SECTION

