MILK OF MAGNESIA MINT
647 GC MOM MINT
f8bba8cb-a111-4285-8bdb-2273a049c3bb
HUMAN OTC DRUG LABEL
Aug 12, 2025
Preferred Pharmaceuticals Inc.
DUNS: 791119022
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
magnesium hydroxide
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (4)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
package Label
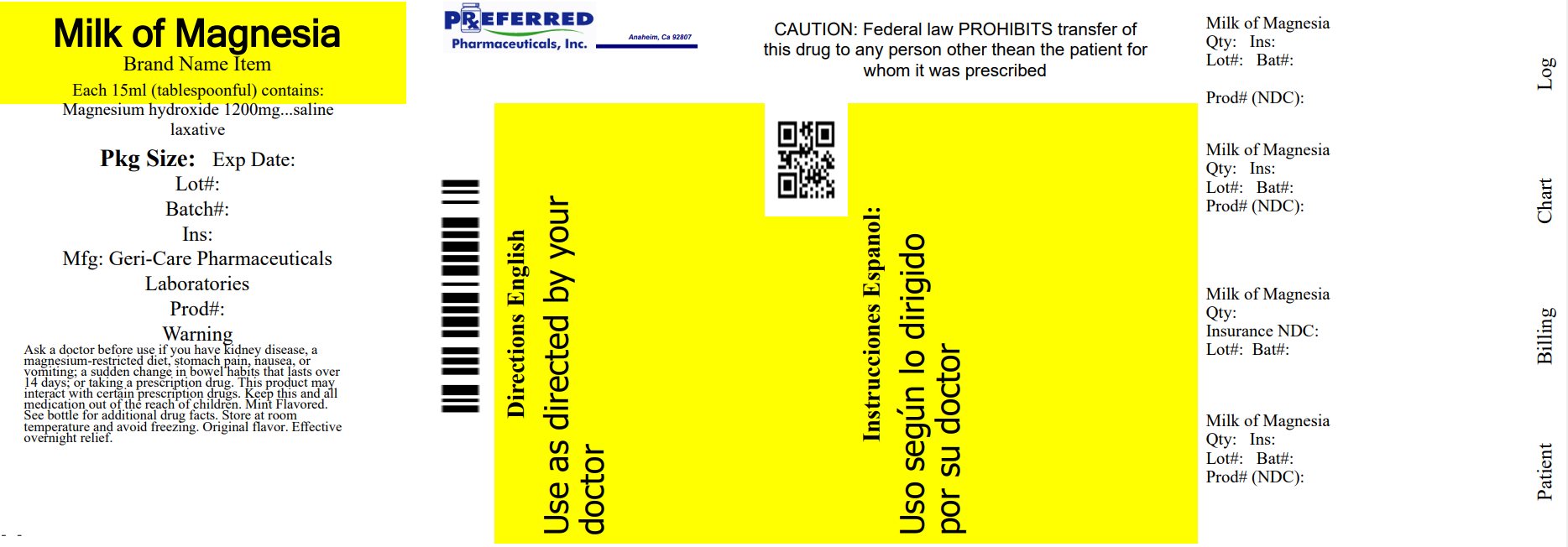
INDICATIONS & USAGE SECTION
Uses
•
relieves occasional constipation (irregularity)
•
generally produces bowel movement in 1/2 to 6 hours
OTC - ACTIVE INGREDIENT SECTION
Active ingredient (in each 15 mL tablespoonful)
Magnesium hydroxide 1200 mg
OTC - PURPOSE SECTION
Purpose
Saline laxative
WARNINGS SECTION
Warnings
Ask a doctor before use if you have
•
kidney disease
•
a magnesium-restricted diet
•
stomach pain, nausea, or vomiting
•
a sudden change in bowel habits that lasts over 14 days
Ask a doctor or pharmacist before use if you aretaking a prescription drug.
This product may interact with certain prescription drugs.
Stop use and ask a doctor if
•
you have rectal bleeding or no bowel movement after using this product. These could be signs of a serious condition.
•
you need to use a laxative for more than 1 week
**If pregnant or breast-feeding,**ask a health professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a
Poison Control Center right away.
DOSAGE & ADMINISTRATION SECTION
Directions
•
shake well before use
•
do not exceed the maximum recommended daily dose in a 24 hour period
•
dose may be taken once a day preferably at bedtime, in divided doses, or as directed by a doctor
•
drink a full glass (8 oz) of liquid with each dose
|
adults and children 12 years and older |
2 to 4 tablespoonfuls |
|
children 6 to 11 years |
1 to 2 tablespoonfuls |
|
children under 6 years |
ask a doctor |
STORAGE AND HANDLING SECTION
Other information
•
**each 15 mL tablespoonful contains:** magnesium 500 mg
•
store at room temperature tightly closed
•
do not freeze
Relabeled By: Preferred Pharmaceuticals Inc.
NDC 68788-8638-3
INACTIVE INGREDIENT SECTION
Inactive ingredients
flavor, purified water, saccharin sodium, sodium hypochlorite
