VENLAFAXINE HYDROCHLORIDE
These highlights do not include all the information needed to use Venlafaxine Extended-Release Tablets safely and effectively. See full prescribing information for Venlafaxine Extended-Release Tablets. Venlafaxine Extended-Release Tablets (venlafaxine hydrochloride) for Oral use Initial U.S. Approval: 1993
68f40801-d35e-464e-ac94-21016b617ea2
HUMAN PRESCRIPTION DRUG LABEL
Oct 29, 2021
XLCare Pharmaceuticals, Inc.
DUNS: 080991142
Products 4
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
venlafaxine
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (15)
venlafaxine
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (15)
venlafaxine
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (15)
venlafaxine
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (15)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL








BOXED WARNING SECTION
5.7 Changes in Weight
Adult Patients: A loss of 5% or more of body weight occurred in 7% of patients treated with venlafaxine hydrochloride extended-release capsules and 2% of placebo-treated patients in the short-term placebo-controlled major depressive disorder trials. The discontinuation rate for weight loss associated with venlafaxine hydrochloride extended-release capsules was 0.1% in major depressive disorder studies. In other placebo-controlled trials, 4% of the patients treated with venlafaxine hydrochloride extended-release capsules and 1% of the placebo-treated patients sustained a loss of 7% or more of body weight during up to 6 months of treatment. None of the patients receiving venlafaxine hydrochloride extended-release capsules in other studies discontinued for weight loss.
The safety and efficacy of venlafaxine therapy in combination with weight loss agents, including phentermine, have not been established. Co-administration of venlafaxine extended-release tablets and weight loss agents is not recommended. Venlafaxine extended-release tablets are not indicated for weight loss alone or in combination with other products.
Pediatric Patients: Weight loss has been observed in pediatric patients (ages 6-17) receiving venlafaxine hydrochloride extended-release capsules. In a pooled analysis of four eight-week, double-blind, placebo-controlled, flexible dose outpatient trials for major depressive disorder (MDD) and another disorder, patients treated with venlafaxine hydrochloride extended-release capsules lost an average of 0.45 kg (n = 333), while placebo-treated patients gained an average of 0.77 kg (n = 333). More patients treated with venlafaxine hydrochloride extended-release capsules than with placebo experienced a weight loss of at least 3.5% in the studies (18% of patients treated with venlafaxine hydrochloride extended-release capsules vs. 3.6% of placebo-treated patients; p<0.001). In a 16-week, double-blind, placebo-controlled, flexible dose outpatient study for another disorder, venlafaxine hydrochloride extended- release capsule-treated patients lost an average of 0.75 kg (n=137), while placebo-treated patients gained an average of 0.76 kg (n=148). More patients treated with venlafaxine hydrochloride extended-release capsules than with placebo experienced a weight loss of at least 3.5% in the study (47% of patients treated with venlafaxine hydrochloride extended-release capsules vs. 14% of placebo-treated patients; p<0.001). Weight loss was not limited to patients with treatment-emergent anorexia [see Warnings and Precautions (5.9)].
The risks associated with longer-term use of venlafaxine hydrochloride extended-release capsules were assessed in an open-label MDD study of children and adolescents who received venlafaxine hydrochloride extended-release capsules for up to six months. The children and adolescents in the study had increases in weight that were less than expected based on data from age-and sex-matched peers. The difference between observed weight gain and expected weight gain was larger for children (<12 years old) than for adolescents (≥12 years old).
INDICATIONS & USAGE SECTION
1 INDICATIONS AND USAGE
1.1 Major Depressive Disorder
Venlafaxine extended-release tablets are indicated for the treatment of major depressive disorder (MDD).
Efficacy of venlafaxine in MDD was shown in both short-term trials and a longer-term trial in MDD [see Clinical Studies (14.1)].
A major depressive episode (DSM-IV) implies a prominent and relatively persistent (nearly every day for at least 2 weeks) depressed mood or the loss of interest or pleasure in nearly all activities, representing a change from previous functioning, and includes the presence of at least five of the following nine symptoms during the same two-week period: depressed mood, markedly diminished interest or pleasure in usual activities, significant change in weight and/or appetite, insomnia or hypersomnia, psychomotor agitation or retardation, increased fatigue, feelings of guilt or worthlessness, slowed thinking or impaired concentration, a suicide attempt or suicidal ideation.
1.2 Social Anxiety Disorder
Venlafaxine extended-release tablets are indicated for the treatment of Social Anxiety Disorder (SAD), also known as Social Phobia, as defined in DSM-IV.
Social Anxiety Disorder (DSM-IV) is characterized by a marked and persistent fear of 1 or more social or performance situations in which the person is exposed to unfamiliar people or to possible scrutiny by others. Exposure to the feared situation almost invariably provokes anxiety, which may approach the intensity of a panic attack. The feared situations are avoided or endured with intense anxiety or distress. The avoidance, anxious anticipation, or distress in the feared situation(s) interferes significantly with the person's normal routine, occupational or academic functioning, or social activities or relationships, or there is a marked distress about having the phobias. Lesser degrees of performance anxiety or shyness generally do not require psychopharmacological treatment.
Efficacy of venlafaxine extended-release in the treatment of SAD was established in short-term SAD trials [see Clinical Studies (14.2)].
Venlafaxine extended-release tablets are a selective serotonin and norepinephrine reuptake inhibitor (SNRI) indicated for: (1)
- Major Depressive Disorder (MDD) (1.1)
- Social Anxiety Disorder (SAD) (1.2)
CONTRAINDICATIONS SECTION
4 CONTRAINDICATIONS
4.1 Monoamine Oxidase Inhibitors (MAOIs)
The use of MAOI’s intended to treat psychiatric disorders with venlafaxine extended-release tablets or within 7 days of stopping treatment with venlafaxine extended-release tablets is contraindicated because of an increased risk of serotonin syndrome. The use of venlafaxine extended-release tablets within 14 days of stopping an MAOI intended to treat psychiatric disorders is also contraindicated. [see Dosage and Administration (2.6) and Warnings and Precautions (5.2)].
Starting venlafaxine extended-release tablets in a patient who is being treated with MAOIs such as linezolid or intravenous methylene blue is also contraindicated because of an increased risk of serotonin syndrome [see Dosage and Administration (2.7) and Warnings and Precautions (5.2)].
- Serotonin Syndrome and MAOIs: Do not use MAOI’s intended to treat psychiatric disorders with venlafaxine extended-release tablets or within 7 days of stopping treatment with venlafaxine extended-release tablets. Do not use venlafaxine extended-release tablets within 14 days of stopping an MAOI intended to treat psychiatric disorders. In addition, do not start venlafaxine extended-release tablets in a patient who is being treated with linezolid or intravenous methylene blue (4.1).
RECENT MAJOR CHANGES SECTION
RECENT MAJOR CHANGES
Warning and Precautions (5.18) 8/2021
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
Venlafaxine extended-release tablets should be administered in a single dose with food either in the morning or in the evening at approximately the same time each day. Each tablet should be swallowed whole with fluid and not divided, crushed, chewed, or placed in water.
2.1 Initial Treatment
Major Depressive Disorder
For most patients, the recommended starting dose for venlafaxine extended- release tablets is 75 mg/day, administered in a single dose. In the clinical trials establishing the efficacy of venlafaxine hydrochloride extended-release capsules in moderately depressed outpatients, the initial dose of venlafaxine was 75 mg/day. For some patients, it may be desirable to start at 37.5 mg/day for 4 to 7 days, to allow new patients to adjust to the medication before increasing to 75 mg/day. While the relationship between dose and antidepressant response for venlafaxine hydrochloride extended-release capsules has not been adequately explored, patients not responding to the initial 75 mg/day dose may benefit from dose increases to a maximum of approximately 225 mg/day. Dose increases should be in increments of up to 75 mg/day, as needed, and should be made at intervals of not less than 4 days, since steady state plasma levels of venlafaxine and its major metabolites are achieved in most patients by day 4. In the clinical trials establishing efficacy, upward titration was permitted at intervals of 2 weeks or more; the average doses were about 140 to 180 mg/day [see Clinical Studies (14)].
It should be noted that, while the maximum recommended dose for moderately depressed outpatients is also 225 mg/day for venlafaxine hydrochloride immediate-release tablets, more severely depressed inpatients in one study of the development program for that product responded to a mean dose of 350 mg/day (range of 150 to 375 mg/day). Whether or not higher doses of venlafaxine extended-release tablets are needed for more severely depressed patients is unknown; however, the experience with venlafaxine hydrochloride extended-release capsule doses higher than 225 mg/day is very limited. [see Warnings and Precautions (5.17)]
Social Anxiety Disorder (Social Phobia)
The recommended dose is 75 mg/day, administered in a single dose. There was no evidence that higher doses confer any additional benefit. [see Warnings and Precautions (5.17)]
2.2 Maintenance Treatment
There is no body of evidence available from controlled trials to indicate how long patients with major depressive disorder should be treated with venlafaxine extended-release tablets.
It is generally agreed that acute episodes of major depressive disorder require several months or longer of sustained pharmacological therapy beyond response to the acute episode. In one study, in which patients responding during 8 weeks of acute treatment with venlafaxine hydrochloride extended- release capsules were assigned randomly to placebo or to the same dose of venlafaxine hydrochloride extended-release capsules (75, 150, or 225 mg/day, qAM) during 26 weeks of maintenance treatment as they had received during the acute stabilization phase, longer-term efficacy was demonstrated. A second longer-term study has demonstrated the efficacy of venlafaxine hydrochloride immediate-release tablets in maintaining a response in patients with recurrent major depressive disorder who had responded and continued to be improved during an initial 26 weeks of treatment and were then randomly assigned to placebo or venlafaxine hydrochloride immediate-release tablets for periods of up to 52 weeks on the same dose (100 to 200 mg/day, on a b.i.d. schedule) [see Clinical Studies (14)]. Based on these limited data, it is not known whether or not the dose of venlafaxine extended-release tablets needed for maintenance treatment is identical to the dose needed to achieve an initial response. Patients should be periodically reassessed to determine the need for maintenance treatment and the appropriate dose for such treatment.
2.3 Special Populations
Treatment of Pregnant Women During the Third Trimester
Neonates exposed to venlafaxine hydrochloride extended-release capsules, other SNRIs, or SSRIs, late in the third trimester have developed complications requiring prolonged hospitalization, respiratory support, and tube feeding [see Use in Specific Populations (8.1)]. When treating pregnant women with venlafaxine extended-release tablets during the third trimester, the physician should carefully consider the potential risks and benefits of treatment.
Patients with Hepatic Impairment
Given the decrease in clearance and increase in elimination half-life for both venlafaxine and ODV that is observed in patients with hepatic cirrhosis and mild and moderate hepatic impairment compared with normal subjects [see Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)], it is recommended that the total daily dose be reduced by 50% in patients with mild to moderate hepatic impairment. Since there was much individual variability in clearance between patients with cirrhosis, it may be necessary to reduce the dose even more than 50%, and individualization of dosing may be desirable in some patients.
Patients with Renal Impairment
Given the decrease in clearance for venlafaxine and the increase in elimination half-life for both venlafaxine and ODV that is observed in patients with renal impairment (GFR = 10 to 70 mL/min) compared with normal subjects [see Use in Specific Populations (8.7) and Clinical Pharmacology (12.3)], it is recommended that the total daily dose be reduced by 25% to 50%.
In patients undergoing hemodialysis, it is recommended that the total daily dose be reduced by 50%. Because there was much individual variability in clearance between patients with renal impairment, individualization of dosage may be desirable in some patients.
Elderly Patients
No dose adjustment is recommended for elderly patients solely on the basis of age. As with any drug for the treatment of major depressive disorder or Social Anxiety Disorder, however, caution should be exercised in treating the elderly. When individualizing the dosage, extra care should be taken when increasing the dose.
2.4 Discontinuing Venlafaxine Extended-Release Tablets
Symptoms associated with discontinuation of venlafaxine hydrochloride extended-release capsules, other SNRI’s, and SSRI’s have been reported [see Warnings and Precautions (5.5)]. Patients should be monitored for these symptoms when discontinuing treatment. A gradual reduction in the dose rather than abrupt cessation is recommended whenever possible. If intolerable symptoms occur following a decrease in the dose or upon discontinuation of treatment, then resuming the previously prescribed dose may be considered. Subsequently, the physician may continue decreasing the dose but at a more gradual rate. In clinical trials with venlafaxine hydrochloride extended- release capsules, tapering was achieved by reducing the daily dose by 75 mg at 1 week intervals. Individualization of tapering may be necessary.
2.5 Switching Patients from Venlafaxine Hydrochloride Immediate-Release
Tablets
Depressed patients who are currently being treated at a therapeutic dose with venlafaxine hydrochloride immediate-release tablets may be switched to venlafaxine extended-release tablets at the nearest equivalent dose (mg/day), e.g., 37.5 mg venlafaxine two-times-a-day to 75 mg venlafaxine extended- release tablets once daily. However, individual dosage adjustments may be necessary.
2.6 Switching a Patient To or From a Monoamine Oxidase Inhibitor (MAOI)
Intended to Treat Psychiatric Disorders
At least 14 days should elapse between discontinuation of an MAOI intended to treat psychiatric disorders and initiation of therapy with venlafaxine extended-release tablets. Conversely, at least 7 days should be allowed after stopping venlafaxine extended-release tablets before starting an MAOI intended to treat psychiatric disorders [see Contraindications (4.1)].
2.7 Use of Venlafaxine Extended-Release Tablets with Other MAOIs, Such as
Linezolid or Methylene Blue
Do not start venlafaxine extended-release tablets in a patient who is being treated with linezolid or intravenous methylene blue because there is increased risk of serotonin syndrome. In a patient who requires more urgent treatment of a psychiatric condition, other interventions, including hospitalization, should be considered [see Contraindications (4.1)].
In some cases, a patient already receiving venlafaxine extended-release tablets therapy may require urgent treatment with linezolid or intravenous methylene blue. If acceptable alternatives to linezolid or intravenous methylene blue treatment are not available and the potential benefits of linezolid or intravenous methylene blue treatment are judged to outweigh the risks of serotonin syndrome in a particular patient, venlafaxine extended- release tablets should be stopped promptly, and linezolid or intravenous methylene blue can be administered. The patient should be monitored for symptoms of serotonin syndrome for 7 days or until 24 hours after the last dose of linezolid or intravenous methylene blue, whichever comes first. Therapy with venlafaxine extended-release tablets may be resumed 24 hours after the last dose of linezolid or intravenous methylene blue [see Warnings and Precautions (5.2)].
The risk of administering methylene blue by non-intravenous routes (such as oral tablets or by local injection) or in intravenous doses much lower than 1 mg/kg with venlafaxine extended-release tablets is unclear. The clinician should, nevertheless, be aware of the possibility of emergent symptoms of serotonin syndrome with such use [see Warnings and Precautions (5.2)].
- Initial Treatment (2.1)
|
** Indication** |
** Starting Dose** |
** Dose Increase** |
** Maximum Dose** |
|
Major Depressive Disorder |
75 mg/day (in some patients, 37.5 mg/day for 4-7 days) |
75 mg/day increments at intervals of 4 days or longer |
225 mg/day |
|
Social Anxiety Disorder |
75 mg/day |
No benefit at higher doses |
75 mg/day |
(2)
- Venlafaxine extended-release tablets should be taken as a single daily dose with food in either the morning or evening at the same time each day. (2)
- Discontinuation: Gradual; individualized as necessary. (2.4)
DOSAGE FORMS & STRENGTHS SECTION
3 DOSAGE FORMS AND STRENGTHS
Venlafaxine extended-release tablets are available as:
- 37.5 mg tablets (White to off white, film coated, round biconvex tablets printed with “392” in black ink)
- 75 mg tablets (White to off white, film coated, round biconvex tablets printed with “393” in black ink)
- 150 mg tablets (White to off white, film coated, round biconvex tablets printed with “394” in black ink)
- 225 mg tablets (White to off white, film coated, round biconvex tablets printed with “395” in black ink)
- 37.5 mg, 75 mg, 150 mg, and 225 mg tablets (3)
DRUG ABUSE AND DEPENDENCE SECTION
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
Venlafaxine extended-release tablets (venlafaxine hydrochloride) are not a controlled substance.
9.2 Abuse
While venlafaxine has not been systematically studied in clinical trials for its potential for abuse, there was no indication of drug-seeking behavior in the clinical trials. However, it is not possible to predict on the basis of premarketing experience the extent to which a CNS active drug will be misused, diverted, and/or abused once marketed. Consequently, physicians should carefully evaluate patients for history of drug abuse and follow such patients closely, observing them for signs of misuse or abuse of venlafaxine (e.g., development of tolerance, incrementations of dose, drug-seeking behavior).
9.3 Dependence
In vitro studies revealed that venlafaxine has virtually no affinity for opiate, benzodiazepine, phencyclidine (PCP), or N-methyl-D-aspartic acid (NMDA) receptors.
Venlafaxine was not found to have any significant CNS stimulant activity in rodents. In primate drug discrimination studies, venlafaxine showed no significant stimulant or depressant abuse liability.
Discontinuation effects have been reported in patients receiving venlafaxine [see Dosage and Administration (2.4) and Warnings and Precautions (5.5)].
OVERDOSAGE SECTION
10 OVERDOSAGE
10.1 Human Experience
Among the patients included in the premarketing evaluation of venlafaxine hydrochloride extended-release capsules, there were 2 reports of acute overdosage with venlafaxine hydrochloride extended-release capsules in major depressive disorder trials, either alone or in combination with other drugs. One patient took a combination of 6 g of venlafaxine hydrochloride extended- release capsules and 2.5 mg of lorazepam. This patient was hospitalized, treated symptomatically, and recovered without any untoward effects. The other patient took 2.85 g of venlafaxine hydrochloride extended-release capsules. This patient reported paresthesia of all four limbs but recovered without sequelae.
There were no reports of acute overdose with venlafaxine hydrochloride extended-release capsules in Social Anxiety Disorder trials.
Among the patients included in the premarketing evaluation with venlafaxine hydrochloride immediate-release tablets, there were 14 reports of acute overdose with venlafaxine, either alone or in combination with other drugs and/or alcohol. The majority of the reports involved ingestion in which the total dose of venlafaxine taken was estimated to be no more than several-fold higher than the usual therapeutic dose. The 3 patients who took the highest doses were estimated to have ingested approximately 6.75 g, 2.75 g, and 2.5 g. The resultant peak plasma levels of venlafaxine for the latter 2 patients were 6.24 and 2.35 μg/mL, respectively, and the peak plasma levels of O-desmethylvenlafaxine were 3.37 and 1.30 μg/mL, respectively. Plasma venlafaxine levels were not obtained for the patient who ingested 6.75 g of venlafaxine. All 14 patients recovered without sequelae. Most patients reported no symptoms. Among the remaining patients, somnolence was the most commonly reported symptom. The patient who ingested 2.75 g of venlafaxine was observed to have 2 generalized convulsions and a prolongation of QTc to 500 msec, compared with 405 msec at baseline. Mild sinus tachycardia was reported in 2 of the other patients.
In postmarketing experience, overdose with venlafaxine has occurred predominantly in combination with alcohol and/or other drugs. The most commonly reported reactions in overdosage include tachycardia, changes in level of consciousness (ranging from somnolence to coma), mydriasis, seizures, and vomiting. Electrocardiogram changes (e.g., prolongation of QT interval, bundle branch block, QRS prolongation), ventricular tachycardia, bradycardia, hypotension, rhabdomyolysis, vertigo, liver necrosis, serotonin syndrome, and death have been reported.
Published retrospective studies report that venlafaxine overdosage may be associated with an increased risk of fatal outcomes compared to that observed with SSRI antidepressant products, but lower than that for tricyclic antidepressants. Epidemiological studies have shown that venlafaxine-treated patients have a higher pre-existing burden of suicide risk factors than SSRI- treated patients. The extent to which the finding of an increased risk of fatal outcomes can be attributed to the toxicity of venlafaxine in overdosage as opposed to some characteristic(s) of venlafaxine-treated patients is not clear. Prescriptions for venlafaxine extended-release tablets should be written for the smallest quantity of tablets consistent with good patient management, in order to reduce the risk of overdose.
10.2 Management of Overdosage
Treatment should consist of those general measures employed in the management of overdosage with any antidepressant.
Ensure an adequate airway, oxygenation, and ventilation. Monitor cardiac rhythm and vital signs. General supportive and symptomatic measures are also recommended. Induction of emesis is not recommended. Gastric lavage with a large bore orogastric tube with appropriate airway protection, if needed, may be indicated if performed soon after ingestion or in symptomatic patients.
Activated charcoal should be administered. Due to the large volume of distribution of this drug, forced diuresis, dialysis, hemoperfusion, and exchange transfusion are unlikely to be of benefit. No specific antidotes for venlafaxine are known.
In managing overdosage, consider the possibility of multiple drug involvement. The physician should consider contacting a poison control center for additional information on the treatment of any overdose. Telephone numbers for certified poison control centers are listed in the Physicians' Desk Reference® (PDR).
DESCRIPTION SECTION
11 DESCRIPTION
Venlafaxine extended-release tablets (venlafaxine hydrochloride) are extended- release tablets for oral administration that contain venlafaxine hydrochloride, a structurally novel antidepressant. Venlafaxine hydrochloride is a selective serotonin and norepinephrine reuptake inhibitor (SNRI). It is designated (R/S)-1-[2-(dimethylamino)-1-(4-methoxyphenyl)ethyl] cyclohexanol hydrochloride or (±)-1-[α-[(dimethylamino)methyl]-p-methoxybenzyl] cyclohexanol hydrochloride and has the empirical formula of C17H27NO2 HCl. Its molecular weight is 313.87. The structural formula is shown below.
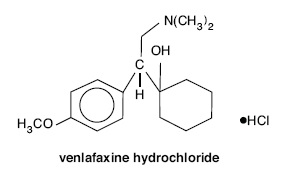
Venlafaxine hydrochloride is a off-white to white crystalline solid with a solubility of 572 mg/mL in water (adjusted to ionic strength of 0.2 M with sodium chloride). Its octanol:water (0.2 M sodium chloride) partition coefficient is 0.43.
Venlafaxine extended-release tablets are formulated as extended-release tablet for once-a-day oral administration. Venlafaxine extended-release tablets use osmotic pressure to deliver venlafaxine hydrochloride at a controlled rate over approximately 24 hours. The system, which resembles a conventional tablet in appearance, comprises an osmotically active core surrounded by a semipermeable membrane. The unitary tablet core is composed of the drug and excipients (including the osmotically active components). There is a precision-laser drilled orifice in the semipermeable membrane on the side of the tablet. In an aqueous environment, such as the gastrointestinal tract, water permeates through the membrane into the tablet core, causing the drug to dissolve and the osmotic components to expand. This expansion pushes the drug out through the orifice. The semipermeable membrane controls the rate at which water permeates into the tablet core, which in turn controls the rate of drug delivery. The controlled rate of drug delivery into the gastrointestinal lumen is thus independent of pH or gastrointestinal motility. The function of venlafaxine extended-release tablets depends on the existence of an osmotic gradient between the contents of the core and the fluid in the gastrointestinal tract. Since the osmotic gradient remains constant, drug delivery remains essentially constant.
The biologically inert components of the tablet remain intact during gastrointestinal transit and are eliminated in the feces as an insoluble shell.
Tablets contain venlafaxine hydrochloride, USP equivalent to 37.5 mg, 75 mg, 150 mg, or 225 mg venlafaxine. Inactive ingredients consist of mannitol, microcrystalline cellulose, povidone, polyethylene glycol, colloidal silicon dioxide, magnesium stearate, cellulose acetate, hypromellose, titanium dioxide and talc.
Each tablet strength also contains black iron oxide, hypromellose and propylene glycol as imprinting ink.
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The mechanism of the antidepressant action of venlafaxine in humans is believed to be associated with its potentiation of neurotransmitter activity in the CNS. Preclinical studies have shown that venlafaxine and its active metabolite, O-desmethylvenlafaxine (ODV), are potent inhibitors of neuronal serotonin and norepinephrine reuptake and weak inhibitors of dopamine reuptake.
12.2 Pharmacodynamics
Venlafaxine and its active metabolite, O-desmethylvenlafaxine (ODV) have no significant affinity for muscarinic cholinergic, H1-histaminergic, or α1-adrenergic receptors in vitro. Pharmacologic activity at these receptors is hypothesized to be associated with the various anticholinergic, sedative, and cardiovascular effects seen with other psychotropic drugs. Venlafaxine and ODV do not possess monoamine oxidase (MAO) inhibitory activity.
12.3 Pharmacokinetics
Steady-state concentrations of venlafaxine and O-desmethylvenlafaxine (ODV) in plasma are attained within 3 days of oral multiple dose therapy. Venlafaxine and ODV exhibited linear kinetics over the dose range of 75 to 450 mg/day. The mean ± SD apparent elimination half-life for venlafaxine and ODV after administration of 75 mg venlafaxine extended-release tablets under fed conditions were 10.7±3.2 hours and 12.5±3.0 hours respectively. Venlafaxine and ODV are minimally bound at therapeutic concentrations to plasma proteins (27% and 30%, respectively).
Absorption and Distribution
Venlafaxine is well absorbed and extensively metabolized in the liver. ODV is the only major active metabolite. On the basis of mass balance studies, at least 92% of a single oral dose of venlafaxine is absorbed. The absolute bioavailability of venlafaxine is about 45%. Administration of 75 mg venlafaxine extended-release tablets under fed conditions resulted in mean ± SD venlafaxine Cmax of 26.9 ± 13.4 ng/mL and AUC of 1536.3 ± 496.8 ng·hr/mL. Tmax was 6.3 ± 2.3 hours. ODV mean ± SD Cmax, AUC, Tmax after administration of 75 mg venlafaxine extended-release tablets under fed conditions were 97.9 ± 29.4 ng/mL, 2926.0 ± 746.1 ng·hr/mL, and 11.6 ± 2.9 hours, respectively.
Administration of venlafaxine hydrochloride extended-release capsules (150 mg q24 hours) generally resulted in lower Cmax (150 ng/mL for venlafaxine and 260 ng/mL for ODV) and later Tmax (5.5 hours for venlafaxine and 9 hours for ODV) than for immediate release venlafaxine tablets (Cmax's for immediate release 75 mg q12 hours were 225 ng/mL for venlafaxine and 290 ng/mL for ODV; Tmax's were 2 hours for venlafaxine and 3 hours for ODV). When equal daily doses of venlafaxine were administered as either an immediate release tablet or the extended-release form of venlafaxine, the exposure to both venlafaxine and ODV would be similar for the two treatments. Venlafaxine extended-release tablets would, therefore, provide a slower rate of absorption, but the same extent of absorption compared with the immediate release tablet.
Food did not affect the pharmacokinetic parameters AUC, Cmax, and Tmax of venlafaxine or its active metabolite, ODV, after administration of venlafaxine extended-release tablets. Time of administration (AM vs PM) would not affect the pharmacokinetics of venlafaxine and ODV.
Equal doses of venlafaxine extended-release tablets are bioequivalent to Effexor XR capsules when administered under fed conditions.
Metabolism and Excretion
Following absorption, venlafaxine undergoes extensive presystemic metabolism in the liver, primarily to ODV, but also to N-desmethylvenlafaxine, N,O-didesmethylvenlafaxine, and other minor metabolites. In vitro studies indicate that the formation of ODV is catalyzed by CYP2D6; this has been confirmed in a clinical study showing that patients with low CYP2D6 levels (“poor metabolizers”) had increased levels of venlafaxine and reduced levels of ODV compared to people with normal CYP2D6 (“extensive metabolizers”). The differences between the CYP2D6 poor and extensive metabolizers, however, are not expected to be clinically important because the sum of venlafaxine and ODV is similar in the two groups and venlafaxine and ODV are pharmacologically approximately equiactive and equipotent.
Approximately 87% of a venlafaxine dose is recovered in the urine within 48 hours as unchanged venlafaxine (5%), unconjugated ODV (29%), conjugated ODV (26%), or other minor inactive metabolites (27%). Renal elimination of venlafaxine and its metabolites is thus the primary route of excretion.
Special Populations
Age and Gender: A population pharmacokinetic analysis of 404 venlafaxine- treated patients from two studies involving both b.i.d. and t.i.d. regimens showed that dose-normalized trough plasma levels of either venlafaxine or ODV were unaltered by age or gender differences. Dosage adjustment based on the age or gender of a patient is generally not necessary [see Dosage and Administration (2)].
Extensive/Poor Metabolizers: Plasma concentrations of venlafaxine were higher in CYP2D6 poor metabolizers than extensive metabolizers. Because the total exposure (AUC) of venlafaxine and ODV was similar in poor and extensive metabolizer groups, however, there is no need for different venlafaxine dosing regimens for these two groups.
Liver Disease: In 9 subjects with hepatic cirrhosis, the pharmacokinetic disposition of both venlafaxine and ODV was significantly altered after oral administration of venlafaxine. Venlafaxine elimination half-life was prolonged by about 30%, and clearance decreased by about 50% in cirrhotic subjects compared to normal subjects. ODV elimination half-life was prolonged by about 60%, and clearance decreased by about 30% in cirrhotic subjects compared to normal subjects. A large degree of intersubject variability was noted. Three patients with more severe cirrhosis had a more substantial decrease in venlafaxine clearance (about 90%) compared to normal subjects.
In a second study, venlafaxine was administered orally and intravenously in normal (n = 21) subjects, and in Child-Pugh A (n = 8) and Child-Pugh B (n = 11) subjects (mildly and moderately impaired, respectively). Venlafaxine oral bioavailability was increased 2 to 3 fold, oral elimination half-life was approximately twice as long and oral clearance was reduced by more than half, compared to normal subjects. In hepatically impaired subjects, ODV oral elimination half-life was prolonged by about 40%, while oral clearance for ODV was similar to that for normal subjects. A large degree of intersubject variability was noted.
Dosage adjustment is necessary in these hepatically impaired patients [see Dosage and Administration (2.3) and Use in Specific Populations (8.6)].
Renal Disease: In a renal impairment study, venlafaxine elimination half-life after oral administration was prolonged by about 50% and clearance was reduced by about 24% in renally impaired patients (GFR=10 to 70 mL/min), compared to normal subjects. In dialysis patients, venlafaxine elimination half-life was prolonged by about 180% and clearance was reduced by about 57% compared to normal subjects. Similarly, ODV elimination half-life was prolonged by about 40% although clearance was unchanged in patients with renal impairment (GFR=10 to 70 mL/min) compared to normal subjects. In dialysis patients, ODV elimination half-life was prolonged by about 142% and clearance was reduced by about 56% compared to normal subjects. A large degree of intersubject variability was noted. Dosage adjustment is necessary in these patients [see Dosage and Administration (2.3) and Use in Specific Populations (8.7)].
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Venlafaxine was given by oral gavage to mice for 18 months at doses up to 120 mg/kg per day, which was 1.7 times the maximum recommended human dose on a mg/m2 basis. Venlafaxine was also given to rats by oral gavage for 24 months at doses up to 120 mg/kg per day. In rats receiving the 120 mg/kg dose, plasma concentrations of venlafaxine at necropsy were 1 times (male rats) and 6 times (female rats) the plasma concentrations of patients receiving the maximum recommended human dose. Plasma levels of the O-desmethyl metabolite were lower in rats than in patients receiving the maximum recommended dose. Tumors were not increased by venlafaxine treatment in mice or rats.
Mutagenesis
Venlafaxine and the major human metabolite, O-desmethylvenlafaxine (ODV), were not mutagenic in the Ames reverse mutation assay in Salmonella bacteria or the Chinese hamster ovary/HGPRT mammalian cell forward gene mutation assay. Venlafaxine was also not mutagenic or clastogenic in the in vitro BALB/c-3T3 mouse cell transformation assay, the sister chromatid exchange assay in cultured Chinese hamster ovary cells, or in the in vivo chromosomal aberration assay in rat bone marrow. ODV was not clastogenic in the in vitro Chinese hamster ovary cell chromosomal aberration assay, but elicited a clastogenic response in the in vivo chromosomal aberration assay in rat bone marrow.
Impairment of Fertility
Reproduction and fertility studies in rats showed no effects on male or female fertility at oral doses of up to 2 times the maximum recommended human dose on a mg/m2 basis.
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
14.1 Major Depressive Disorder
The efficacy of venlafaxine hydrochloride extended-release capsules as a treatment for major depressive disorder was established in two placebo- controlled, short-term, flexible-dose studies in adult outpatients meeting DSM-III-R or DSM-IV criteria for major depressive disorder.
A 12-week study utilizing venlafaxine hydrochloride extended-release capsules doses in a range 75 to 150 mg/day (mean dose for completers was 136 mg/day) and an 8-week study utilizing venlafaxine hydrochloride extended-release capsules doses in a range 75 to 225 mg/day (mean dose for completers was 177 mg/day) both demonstrated superiority of venlafaxine hydrochloride extended- release capsules over placebo on the HAM-D total score, HAM-D Depressed Mood Item, the MADRS total score, the Clinical Global Impressions (CGI) Severity of Illness item, and the CGI Global Improvement item. In both studies, venlafaxine hydrochloride extended-release capsules were also significantly better than placebo for certain factors of the HAM-D, including the anxiety/somatization factor, the cognitive disturbance factor, and the retardation factor, as well as for the psychic anxiety score.
A 4-week study of inpatients meeting DSM-III-R criteria for major depressive disorder with melancholia utilizing venlafaxine hydrochloride immediate- release tablets in a range of 150 to 375 mg/day (t.i.d. schedule) demonstrated superiority of venlafaxine hydrochloride immediate-release tablets over placebo. The mean dose in completers was 350 mg/day.
Examination of gender subsets of the population studied did not reveal any differential responsiveness on the basis of gender.
In one longer-term study, adult outpatients meeting DSM-IV criteria for major depressive disorder who had responded during an 8-week open trial on venlafaxine hydrochloride extended-release capsules (75, 150, or 225 mg, qAM) were randomized to continuation of their same venlafaxine hydrochloride extended-release capsules dose or to placebo, for up to 26 weeks of observation for relapse.
Response during the open phase was defined as a CGI Severity of Illness item score of ≤3 and a HAM-D-21 total score of ≤10 at the day 56 evaluation. Relapse during the double-blind phase was defined as follows: (1) a reappearance of major depressive disorder as defined by DSM-IV criteria and a CGI Severity of Illness item score of ≥4 (moderately ill), (2) 2 consecutive CGI Severity of Illness item scores of ≥4, or (3) a final CGI Severity of Illness item score of ≥4 for any patient who withdrew from the study for any reason. Patients receiving continued venlafaxine hydrochloride extended- release capsules treatment experienced significantly lower relapse rates over the subsequent 26 weeks compared with those receiving placebo.
In a second longer-term trial, adult outpatients meeting DSM-III-R criteria for major depressive disorder, recurrent type, who had responded (HAM-D-21 total score ≤12 at the day 56 evaluation) and continued to be improved [defined as the following criteria being met for days 56 through 180: (1) no HAM-D-21 total score ≥20; (2) no more than 2 HAM-D-21 total scores >10, and (3) no single CGI Severity of Illness item score ≥4 (moderately ill)] during an initial 26 weeks of treatment on venlafaxine hydrochloride immediate- release tablets (100 to 200 mg/day, on a b.i.d. schedule) were randomized to continuation of their same dose of venlafaxine hydrochloride immediate-release tablets or to placebo. The follow-up period to observe patients for relapse, defined as a CGI Severity of Illness item score ≥4, was for up to 52 weeks. Patients receiving continued treatment with venlafaxine hydrochloride immediate-release tablets experienced significantly lower relapse rates over the subsequent 52 weeks compared with those receiving placebo.
14.2 Social Anxiety Disorder (Social Phobia)
The efficacy of venlafaxine hydrochloride extended-release capsules as a treatment for Social Anxiety Disorder (also known as Social Phobia) was established in two double-blind, parallel group, 12-week, multicenter, placebo-controlled, flexible-dose studies in adult outpatients meeting DSM-IV criteria for Social Anxiety Disorder. Patients received doses in a range of 75 to 225 mg/day. Efficacy was assessed with the Liebowitz Social Anxiety Scale (LSAS). In these two trials, venlafaxine hydrochloride extended-release capsules were significantly more effective than placebo on change from baseline to endpoint on the LSAS total score.
Examination of subsets of the population studied did not reveal any differential responsiveness on the basis of gender. There was insufficient information to determine the effect of age or race on outcome in these studies.
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
Venlafaxine extended-release tablets 37.5 mg are white to off white, film coated, round biconvex tablets printed with “392” in black ink. They are supplied as follows:
Unit of Use Bottles of 30 Tablets NDC 72865-197-30
Unit of Use Bottles of 90 Tablets NDC 72865-197-90
Venlafaxine extended-release tablets 75 mg are white to off white, film coated, round biconvex tablets printed with “393” in black ink. They are supplied as follows:
Unit of Use Bottles of 30 Tablets NDC 72865-198-30
Unit of Use Bottles of 90 Tablets NDC 72865-198-90
Venlafaxine extended-release tablets 150 mg are white to off white, film coated, round biconvex tablets printed with “394” in black ink. They are supplied as follows:
Unit of Use Bottles of 30 Tablets NDC 72865-199-30
Unit of Use Bottles of 90 Tablets NDC 72865-199-90
Venlafaxine extended-release tablets 225 mg are white to off white, film coated, round biconvex tablets printed with “395” in black ink. They are supplied as follows:
Unit of Use Bottles of 30 Tablets NDC 72865-200-30
Unit of Use Bottles of 90 Tablets NDC 72865-200-90
Store at 25°C (77°F); excursions permitted to 15-30°C (59-86°F)[see USP Controlled Room Temperature]. Protect from moisture and humidity.
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Prescribers or other health professionals should inform patients, their families, and their caregivers about the benefits and risks associated with treatment with venlafaxine extended-release tablets and should counsel them in its appropriate use. A patient Medication Guide about “Antidepressant Medicines, Depression and Other Serious Mental Illness, and Suicidal Thoughts or Actions” is available for venlafaxine extended-release tablets. The prescriber or health professional should instruct patients, their families, and their caregivers to read the Medication Guide and should assist them in understanding its contents. Patients should be given the opportunity to discuss the contents of the Medication Guide and to obtain answers to any questions they may have. The complete text of the Medication Guide is reprinted at the end of this document.
Patients should be advised of the following issues and asked to alert their prescriber if these occur while taking venlafaxine extended-release tablets.
17.1 Clinical Worsening and Suicide Risk
Patients, their families, and their caregivers should be encouraged to be alert to the emergence of anxiety, agitation, panic attacks, insomnia, irritability, hostility, aggressiveness, impulsivity, akathisia (psychomotor restlessness), hypomania, mania, other unusual changes in behavior, worsening of depression, and suicidal ideation, especially early during antidepressant treatment and when the dose is adjusted up or down. Families and caregivers of patients should be advised to look for the emergence of such symptoms on a day-to-day basis, since changes may be abrupt. Such symptoms should be reported to the patient's prescriber or health professional, especially if they are severe, abrupt in onset, or were not part of the patient's presenting symptoms. Symptoms such as these may be associated with an increased risk for suicidal thinking and behavior and indicate a need for very close monitoring and possibly changes in the medication.
17.2 Interference with Cognitive and Motor Performance
Clinical studies were performed to examine the effects of venlafaxine on behavioral performance of healthy individuals. The results revealed no clinically significant impairment of psychomotor, cognitive, or complex behavior performance. However, since any psychoactive drug may impair judgment, thinking, or motor skills, patients should be cautioned about operating hazardous machinery, including automobiles, until they are reasonably certain that venlafaxine therapy does not adversely affect their ability to engage in such activities.
17.3 Concomitant Medication
Patients should be advised to inform their physicians if they are taking, or plan to take, any prescription or over-the-counter drugs, including herbal preparations and nutritional supplements, since there is a potential for interactions.
Patients should be cautioned about the risk of serotonin syndrome with the concomitant use of venlafaxine extended-release tablets and triptans, tricyclic antidepressants, fentanyl, lithium, tramadol, amphetamines, tryptophan, buspirone, and St. John’s Wort supplements or other serotonergic agents [see Warnings and Precautions (5.2) and Drug Interactions (7.10)].
Patients should be cautioned about the concomitant use of venlafaxine extended-release tablets and NSAID’s, aspirin, warfarin, or other drugs that affect coagulation since combined use of psychotropic drugs that interfere with serotonin reuptake and these agents has been associated with an increased risk of bleeding [see Warnings and Precautions (5.13) and Drug Interactions (7.11)].
17.4 Alcohol
Although venlafaxine has not been shown to increase the impairment of mental and motor skills caused by alcohol, patients should be advised to avoid alcohol while taking venlafaxine.
17.5 Allergic Reactions
Patients should be advised to notify their physician if they develop a rash, hives, or a related allergic phenomenon.
17.6 Pregnancy
Patients should be advised to notify their physician if they become pregnant or intend to become pregnant during therapy.
17.7 Nursing
Patients should be advised to notify their physician if they are breast- feeding an infant.
17.8 Angle Closure Glaucoma
Patients should be advised that taking venlafaxine can cause mild pupillary dilation, which in susceptible individuals, can lead to an episode of angle closure glaucoma. Pre-existing glaucoma is almost always open-angle glaucoma because angle closure glaucoma, when diagnosed, can be treated definitively with iridectomy. Open-angle glaucoma is not a risk factor for angle closure glaucoma. Patients may wish to be examined to determine whether they are susceptible to angle closure, and have a prophylactic procedure (e.g., iridectomy), if they are susceptible. [see Warnings and Precautions (5.4)]
17.9 Sexual Dysfunction
Advise patient that use of venlafaxine extended-release tablets may cause symptoms of sexual dysfunction in both male and female patients. Inform patients that they should discuss any changes in sexual function and potential management strategies with their healthcare provider [see Warnings and Precautions (5.18)].
Manufactured by:
Ascent Pharmaceuticals, Inc.
Central Islip, NY 11722
Manufactured for:
XLCare Pharmaceuticals, Inc.
242 South Culver Street, Suite 202
Lawrenceville, GA 30046
Rev: 10/21
SPL MEDGUIDE SECTION
MEDICATION GUIDE
Venlafaxine Extended-Release Tablets
(ven-luh-fak-seen)
Antidepressant Medicines, Depression and Other Serious Mental Illnesses, and Suicidal Thoughts or Actions
Read the Medication Guide that comes with your or your family member’s antidepressant medicine. This Medication Guide is only about the risk of suicidal thoughts and actions with antidepressant medicines.Talk to your, or your family member’s, healthcare provider about:
- all risks and benefits of treatment with antidepressant medicines
- all treatment choices for depression or other serious mental illness
Who should not take venlafaxine extended-release tablets?
- take a monoamine oxidase inhibitor (MAOI). Ask your healthcare provider or pharmacist if you are not sure if you take an MAOI, including the antibiotic linezolid.
- Do not take an MAOI within 7 days of stopping venlafaxine extended-release tablets unless directed to do so by your physician.
- Do not start venlafaxine extended-release tablets if you stopped taking an MAOI in the last 2 weeks unless directed to do so by your physician.
What is the most important information I should know about antidepressant medicines, depression and other serious mental illnesses, and suicidal thoughts or actions?
1. Antidepressant medicines may increase suicidal thoughts or actions in some children, teenagers, and young adults within the first few months of treatment.
**2. Depression and other serious mental illnesses are the most important causes of suicidal thoughts and actions. Some people may have a particularly high risk of having suicidal thoughts or actions.**These include people who have (or have a family history of) bipolar illness (also called manic- depressive illness) or suicidal thoughts or actions.
3. How can I watch for and try to prevent suicidal thoughts and actions in myself or a family member?
- Pay close attention to any changes, especially sudden changes, in mood, behaviors, thoughts, or feelings. This is very important when an antidepressant medicine is started or when the dose is changed.
- Call the healthcare provider right away to report new or sudden changes in mood, behavior, thoughts, or feelings.
- Keep all follow-up visits with the healthcare provider as scheduled. Call the healthcare provider between visits as needed, especially if you have concerns about symptoms.
Call a healthcare provider right away if you or your family member has any of the following symptoms, especially if they are new, worse, or worry you:

What else do I need to know about antidepressant medicines?
***Never stop an antidepressant medicine without first talking to a healthcare provider.**Stopping an antidepressant medicine suddenly can cause other symptoms.
***Visual Problems.**Only some people are at risk for these problems. You may want to undergo an eye examination to see if you are at risk and receive preventative treatment if you are. ***Antidepressants are medicines used to treat depression and other illnesses.**It is important to discuss all the risks of treating depression and also the risks of not treating it. Patients and their families or other caregivers should discuss all treatment choices with the healthcare provider, not just the use of antidepressants. ***Antidepressant medicines have other side effects.**Talk to the healthcare provider about the side effects of the medicine prescribed for you or your family member. ***Antidepressant medicines can interact with other medicines.**Know all of the medicines that you or your family member takes. Keep a list of all medicines to show the healthcare provider. Do not start new medicines without first checking with your healthcare provider. ***Not all antidepressant medicines prescribed for children are FDA approved for use in children.**Talk to your child’s healthcare provider for more information. ***Sexual problems (dysfunction).**Taking serotonin and norepinephrine reuptake inhibitors (SNRIs), including venlafaxine extended-release tablets, may cause sexual problems.
Symptoms in males may include:
o Delayed ejaculation or inability to have an ejaculation
o Decreased sex drive
o Problems getting or keeping an erection
Symptoms in females may include:
o Decreased sex drive
o Delayed orgasm or inability to have an orgasm
Talk to your healthcare provider if you develop any changes in your sexual function or if you have any questions or concerns about sexual problems during treatment with venlafaxine extended-release tablets. There may be treatments your healthcare provider can suggest.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
This Medication Guide has been approved by the U.S. Food and Drug Administration for all antidepressants.
Manufactured by:
Ascent Pharmaceuticals, Inc.
Central Islip, NY 11722
Manufactured for:
XLCare Pharmaceuticals, Inc.
242 South Culver Street, Suite 202
Lawrenceville, GA 30046
Rev: 10/21
