TRYPTYR
These highlights do not include all the information needed to use TRYPTYR safely and effectively. See full prescribing information for TRYPTYR . TRYPTYR (acoltremon ophthalmic solution) 0.003%, for topical ophthalmic use Initial U.S. Approval: 2025
2b7715a1-035c-4002-b582-5238efee5d58
HUMAN PRESCRIPTION DRUG LABEL
Jul 30, 2025
Alcon Laboratories, Inc.
DUNS: 008018525
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Acoltremon
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
Drug Labeling Information
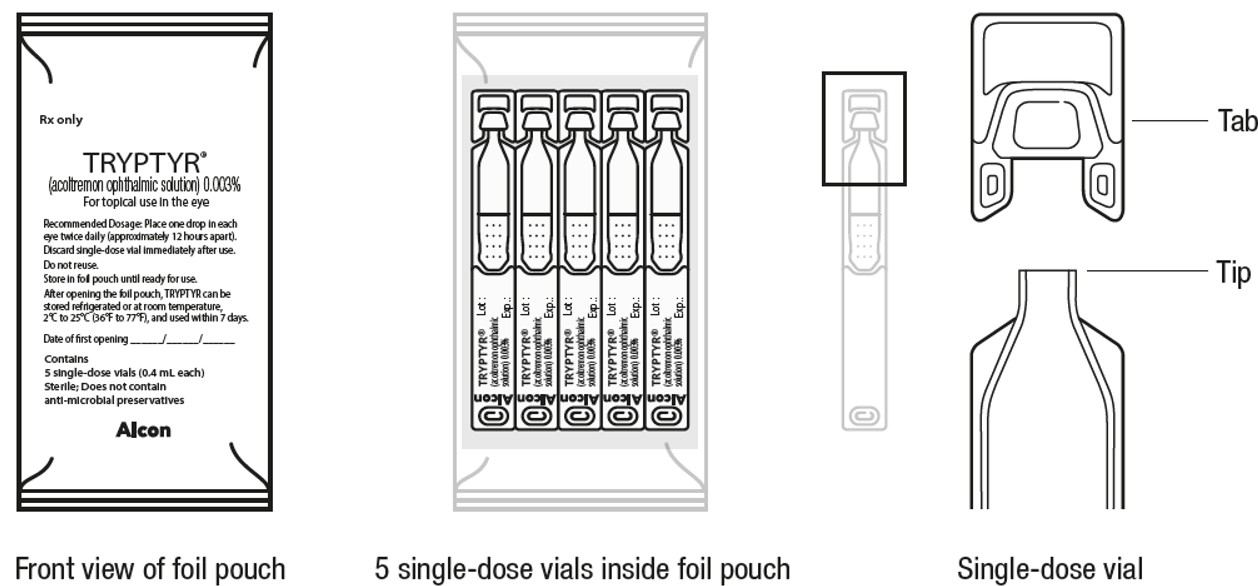
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
TRYPTYR**®**
(acoltremon ophthalmic
solution) 0.003%
3390
Alcon
Lot:
Exp.:
NDC 0065-8595-02 441121
Rx only
TRYPTYR**®**
(acoltremon ophthalmic solution) 0.003%
For topical use in eye
Recommended Dosage: Place one drop in each
eye twice daily (approximately 12 hours apart).
Discard single-dose vial immediately after use.
Do not reuse.
Store in foil pouch until ready for use.
After opening the foil pouch, TRYPTYR can be
stored refrigerated or at room temperature,
2°C to 25°C (36°F to 77°F), and used within 7 days.
Date of first opening //_____
Contains
5 single-use vials (0.4 mL each)
Sterile; Does not contain
****anti-microbial preservatives
Alcon
LOT: ####A
EXP.: YYYY-MM
Manufactured for:
Alcon Laboratories, Inc.
Fort Worth, TX 76134 USA
NDC 0065-8595-02
Rx only
TRYPTYR**®**
(acoltremon ophthalmic solution) 0.003%
For topical application in the eye
60 SINGLE-DOSE VIALS ● 30-Day SUPPLY
Does not contain anti-microbial preservatives
Sterile
Alcon
CONTAINS
12 pouches x 5 single-dose
vials (0.4 mL each)
Each mL contains:
** Active:** acoltremon 0.003%
Inactives: polyoxyl 35 castor oil,
sodium dihydrogen phosphate
dihydrate, sodium chloride,
hypromellose, sodium hydroxide
to adjust pH, and purified water.
STORAGE
Store unopened carton in the
refrigerator, 2°C to 8°C
(36°F to 46°F). After opening,
TRYPTYR can be stored
refrigerated or at room
temperature up to 30 days in the
unopened foil pouch,
2°C to 25°C (36°F to 77°F).
Date carton first opened
//___
See additional discard
instructions on the pouch.
DOSING
Recommended Dosage:
Place one drop in each eye twice
daily (approximately 12 hours
apart).
To avoid contamination, do not
touch tip of container to any
surface. Use one single-dose vial
immediately after opening and
then discard. Do not reuse.
Please see full Prescribing
Information and Instructions for
Use, inside carton, for additional
dosage, storage and safety
information.
Manufactured in France for
Alcon Laboratories, Inc.
Fort Worth, TX 76134 USA
Product of India
U.S. Pat.: www.alconpatents.com
NDC 0065-8595-02
GTIN: 00300658595027
LOT: ####A
EXP.: YYYY-MM
SN: 01234567891234
TRYPTYR**®**
(acoltremon ophthalmic solution) 0.003%
439701
INDICATIONS & USAGE SECTION
1 INDICATIONS AND USAGE
TRYPTYR is indicated for the treatment of the signs and symptoms of dry eye disease.
TRYPTYR is a TRPM8 thermoreceptor agonist indicated for the treatment of the signs and symptoms of dry eye disease. (1)
CONTRAINDICATIONS SECTION
4 CONTRAINDICATIONS
None.
None. (4)
WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Potential for Eye Injury and Contamination
To avoid the potential for eye injury and contamination, do not touch the vial tip to the eye or other surfaces.
5.2 Use with Contact Lenses
TRYPTYR should not be administered while wearing contact lenses. If contact lenses are worn, they should be removed prior to administration of the solution. Lenses may be reinserted 15 minutes following administration of TRYPTYR.
To avoid the potential for eye injury and contamination, do not touch the vial tip to the eye or other surfaces. (5.1)
ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In patients with dry eye disease, 766 patients received at least one dose of TRYPTYR in four randomized controlled clinical trials across 71 sites in the United States. The most common ocular adverse reaction observed in controlled clinical studies with TRYPTYR was instillation site pain (50%). Less than 1% of patients discontinued therapy due to burning or stinging sensation in the eyes.
The most common adverse reaction was instillation site pain (50%). (6.1)
**To report SUSPECTED ADVERSE REACTIONS, contact Alcon Laboratories, Inc. at 1-800-757-9780, or FDA at 1-800-FDA-1088 or **www.fda.gov/medwatch.
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
Instill one drop in each eye twice daily (approximately 12 hours apart).
2.2 Administration Instructions
Wash hands before use.
The single-dose vials are to be used immediately after opening and can be used to dose both eyes. Discard the single-dose vial, including any remaining contents, immediately after use.
TRYPTYR can be used concomitantly with other topical ophthalmic eye drops. If more than one topical ophthalmic drug is being used, the drugs should be administered at least five (5) minutes apart.
Contact lenses should be removed prior to the administration of TRYPTYR and may be reinserted 15 minutes following administration.
If one dose is missed, treatment should continue with the next dose.
Instill one drop in each eye twice daily (approximately 12 hours apart). (2)
DOSAGE FORMS & STRENGTHS SECTION
3 DOSAGE FORMS AND STRENGTHS
TRYPTYR is a clear to slightly opalescent, colorless ophthalmic solution containing 0.003% acoltremon in a single-dose vial.
Ophthalmic solution: 0.003% acoltremon in a single-dose vial. (3)
USE IN SPECIFIC POPULATIONS SECTION
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no adequate and well-controlled studies on TRYPTYR in pregnant women. Systemic exposure to acoltremon from ocular administration is negligible [see Clinical Pharmacology (12.3)]. Intravenous administration of acoltremon to pregnant rats and rabbits during organogenesis did not produce embryofetal toxicity at 806- and 2151-fold the maximum recommended human ocular dose (MRHOD) of acoltremon on a mg/m2 basis (see Data).
All pregnancies have a risk of birth defect, loss, or other adverse outcomes. In the US general population, the estimated background risk of major birth defects is 2 to 4%, and of miscarriage is 15 to 20%, of clinically recognized pregnancies.
Data
Animal Data
In embryofetal developmental studies in pregnant rats and rabbits dosed by intravenous injection daily during organogenesis from gestation days 6-17 and gestation days 7-19, respectively, no maternal or fetal toxicity was observed at 806- and 2151-fold the MRHOD of acoltremon on a mg/m2 basis.
8.2 Lactation
Risk Summary
There are no data on the presence of acoltremon in human milk, the effects on the breastfed infant, or the effects on milk production. However, systemic exposure to acoltremon following topical ocular administration is low. The lack of clinical data during lactation precludes a clear determination of the risk of TRYPTYR to an infant during lactation; however, the developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for TRYPTYR.
8.4 Pediatric Use
The safety and effectiveness of TRYPTYR have not been established in pediatric patients.
8.5 Geriatric Use
No clinically relevant differences in safety have been observed between elderly and younger patients.
DESCRIPTION SECTION
11 DESCRIPTION
TRYPTYR (acoltremon ophthalmic solution) 0.003% contains an agonist of TRPM8 ion channels. Acoltremon’s chemical name is (1R,2S,5R)-2-isopropyl-N-(4-methoxyphenyl)-5-methylcyclohexane-1-carboxamide. The molecular formula of acoltremon is C18H27NO2 and has a molecular weight of 289.42 g/mol.
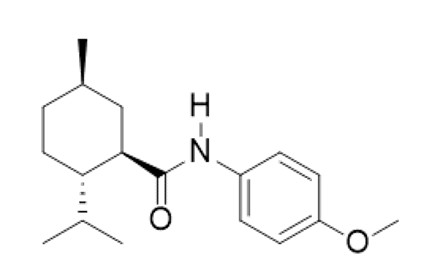
Acoltremon is a white to pale yellow crystalline solid, that is insoluble in water.
TRYPTYR is a sterile, clear to slightly opalescent, colorless, isotonic aqueous solution for topical ophthalmic use, with a pH of approximately 7 and an osmolality of 280 to 330 mOsm/kg. Each mL of TRYPTYR contains active: acoltremon 0.003%; and inactives: polyoxyl 35 castor oil, sodium dihydrogen phosphate dihydrate, sodium chloride, hypromellose and purified water. Additionally, sodium hydroxide is used to adjust pH. TRYPTYR does not contain an anti-microbial preservative.
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Studies in animals suggest that acoltremon, the active substance in TRYPTYR, is an agonist of transient receptor potential melastatin 8 (TRPM8) thermoreceptors. TRPM8 thermoreceptor stimulation has been shown to activate trigeminal nerve signaling leading to increased basal tear production. The exact mechanism of action for TRYPTYR in dry eye disease is unknown.
12.3 Pharmacokinetics
PK was assessed in 25 patients with dry eye disease receiving TRYPTYR administration (1 drop twice daily) on Days 1, 14, and 90. A total of three (3) (12.0%) had plasma concentrations above 20 pg/mL (the lower limit of quantification), with the highest plasma concentration of 213 pg/mL.
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity
Long term studies in animals have not been performed to evaluate the
carcinogenic potential of acoltremon.
Mutagenicity
Acoltremon was not mutagenic or clastogenic in the standard battery of
genotoxicity tests including a bacterial reverse mutation assay, an in vitro
chromosomal aberration assay in human peripheral lymphocytes and micronucleus
assay in rats.
Impairment of Fertility
Studies to evaluate the potential effects of acoltremon on male or female
fertility in animals have not been performed.
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
The efficacy of TRYPTYR for the treatment of dry eye disease was supported by two randomized, multi-center, double-masked, vehicle-controlled studies (COMET-2 [NCT-05285644] and COMET-3 [NCT-05360966]) enrolling a total of 931 dry eye patients (462 of which received TRYPTYR).
Patients were randomized to TRYPTYR or vehicle (placebo) in a 1:1 ratio and dosed twice a day for 90 days. Use of artificial tears was not allowed during the studies. The mean age was 61 years (range, 30-93 years). The majority of patients were female (74.8%). Enrollment criteria included signs (i.e., corneal fluorescein staining score [2-15] and anesthetized Schirmer tear test [2-9 mm]) and symptoms (i.e., SANDE Score [≥ 50] and Ocular Discomfort Score [≥ 50]) of dry eye disease.
Efficacy
Tear film production was measured by unanesthetized Schirmer tear test assessed using a Schirmer strip (0 - 35 mm). The average baseline unanesthetized Schirmer scores for TRYPTYR and Vehicle treated patients was 6.2 mm and 5.9 mm in the COMET-2 study, and 6.8 mm and 6.4 mm in the COMET-3 study, respectively. Of the patients treated at Day 14 (primary endpoint) with TRYPTYR, 42.6% achieved ≥ 10 mm increase in Schirmer score from baseline in the COMET-2 study and 53.2% achieved ≥ 10 mm increase in Schirmer score from baseline at Day 14 in the COMET-3 study, compared to 8.2% and 14.4% of vehicle-treated patients in the COMET-2 study and the COMET-3 study, respectively. A statistically significant improvement in tear production favoring TRYPTYR (p<0.01) was observed in both studies (Table 1).
Table 1: Percent of Patients Achieving ≥ 10 mm Improvement from Baseline in Schirmer Score at Day 14 in Patients with Dry Eye Disease|
Tear Production | ||||
|
COMET-2 |
COMET-3 | |||
|
TRYPTYR |
Vehicle |
TRYPTYR |
Vehicle | |
|
≥ 10 mm increase in tear production at Day 14 |
42.6% |
8.2% |
53.2% |
14.4% |
|
Difference (95% CI) |
34.4% (26.9, 42.0) |
38.8% (30.8, 46.8) | ||
|
P-value versus vehicle |
< 0.01 |
< 0.01 |
Consistent results were observed at all timepoints through Day 90.
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
TRYPTYR (acoltremon ophthalmic solution) 0.003% is supplied as a sterile, clear to slightly opalescent, and colorless solution in a low-density polyethylene (LDPE), single-dose vial with a 0.4 mL fill. One strip of 5 single-dose vials is packaged in a foil pouch with twelve (12) pouches in a carton.
NDC 0065-8595-02; Carton of 60 Single-Dose Vials.
Storage
****Store refrigerated at 2°C to 8°C (36°F to 46°F). After opening the carton, TRYPTYR may be stored refrigerated or at room temperature at 2°C to 25°C (36°F to 77°F). If stored at room temperature, TRYPTYR should be used within 30 days, not to exceed the expiration date printed on the carton and foil pouch.
After opening each foil pouch, the single-dose vials should be used within 7 days, not to exceed the expiration date printed on the vial. Store unopened single-dose vials in the original foil pouch until ready to use.
PATIENT COUNSELING INFORMATION
17 PATIENT COUNSELING INFORMATION
Advise patients to read the FDA-approved patient labeling (Instructions for Use).
Potential for Eye Injury and Contamination
Advise patients not to touch the tip of the single-dose vial to their eye or
to any surface in order to avoid eye injury or contamination of the solution
[see Warnings and Precautions (5.1)].
Advise patients that one single-dose vial can be used to dose both eyes immediately after opening. Discard the single-dose vial, including any remaining contents, after use [see Dosage and Administration (2)].
Use With Contact Lenses
Advise patients that contact lenses should be removed prior to administration
of TRYPTYR and can be reinserted 15 minutes after administration [see Warnings and Precautions (5.2)].
Use with Other Ophthalmic Drugs
Advise patients that if more than one topical ophthalmic drug is being used,
the drugs should be administered at least five (5) minutes apart [see Dosage and Administration (2)].
Storage Information
Instruct patients to store unopened single-dose vials in the original foil
pouch until ready to use [see How Supplied/Storage and Handling (16)].
Manufactured for Alcon Laboratories, Inc.
Fort Worth, TX 76134, USA.
U.S. Pat.: www.alconpatents.com
©2025 Alcon Inc.
SPL PATIENT PACKAGE INSERT SECTION
INSTRUCTIONS FOR USE SECTION
|
INSTRUCTIONS FOR USE |
|
This Instructions for Use contains information on how to use TRYPTYR. Read this Instructions for Use before you start using TRYPTYR and each time you get a refill. There may be new information. This leaflet does not take the place of talking to your healthcare provider about your medical condition or your treatment. |
|
|
|
Important Information You Need to Know Before UsingTRYPTYR****:
|
|
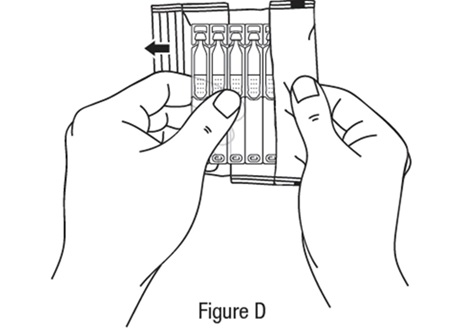
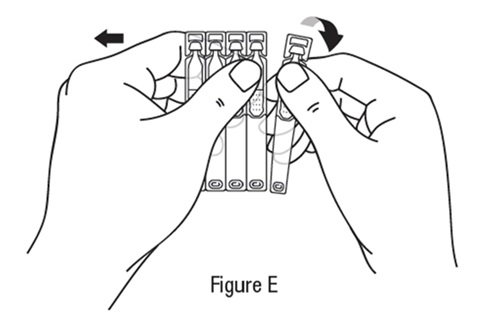
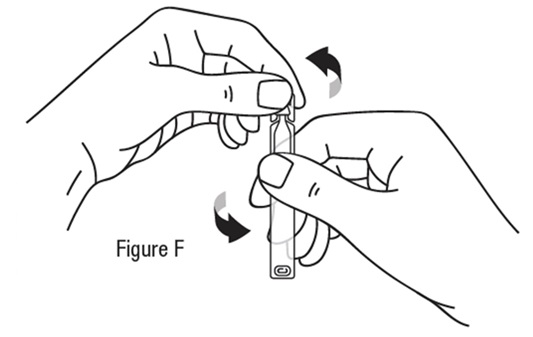
Follow steps 1 to 15 each time you use TRYPTYR
Step 2
|
|
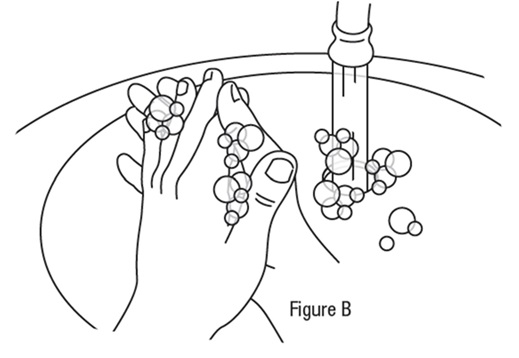
Preparing a single-use vial *Wash your hands before each use to make sure you do not infect your eyes (see Figure B).
Step 5
|
|
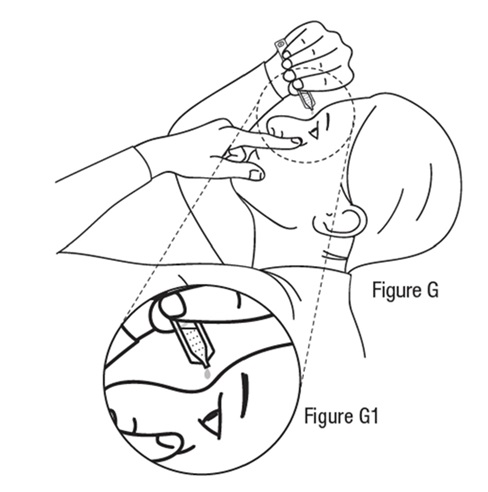
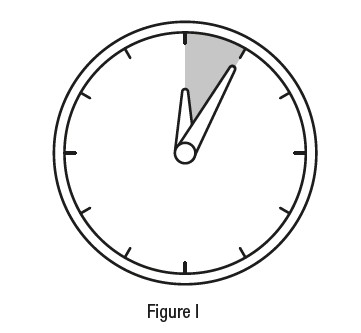
Applying a dose ofTRYPTYR**** to your eyes
Step 9
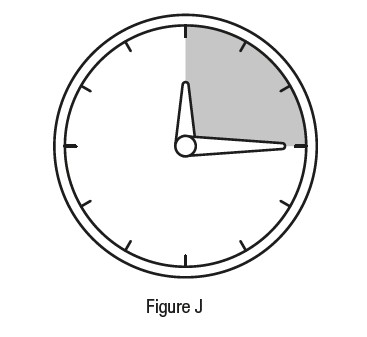
Step 10
*Do not allow the tip of the vial to touch your eye to avoid eye injury or infection. Step 11
*Repeat Steps 8 through 11 for your other eye.
|
|
After dosing and disposal instructions
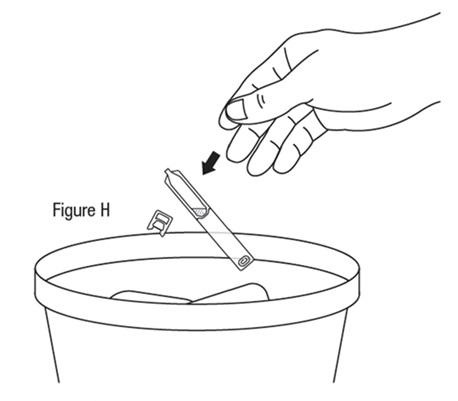
*Do not reuse vial.
Step 15
|
|
How should I store TRYPTYR?
|
|
Manufactured for: Alcon Laboratories, Inc. Fort Worth, TX 76134, U.S.A. |
This Instructions for Use has been approved by the U.S. Food and Drug Administration. 5/2025