Lanthanum Carbonate
These highlights do not include all the information needed to use LANTHANUM CARBONATE CHEWABLE TABLETS safely and effectively. See full prescribing information for LANTHANUM CARBONATE CHEWABLE TABLETS. LANTHANUM CARBONATE Chewable Tablets, for oral use Initial U.S. Approval: 2004
91556a42-5e16-4f86-bae9-a8cdd6b182a6
HUMAN PRESCRIPTION DRUG LABEL
Jan 22, 2024
Lupin Pharmaceuticals, Inc.
DUNS: 089153071
Products 3
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Lanthanum Carbonate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
Lanthanum Carbonate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
Lanthanum Carbonate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Lanthanum Carbonate Chewable Tablets 1000 mg - Bottle of 10 Tablets
Label artwork:
NDC 68180-821-10
Bottle of 10 tablets
Each chewable tablet contains 1000 mg of lanthanum as lanthanum carbonate
dihydrate


** Carton artwork:**
****NDC 68180-821-47
Each chewable tablet contains 1000 mg of lanthanum as lanthanum carbonate
dhydrate
PATIENT PACK ONE MONTH SUPPLY
ATTENTION PHARMACIST: Each patient is required to receive the enclosed
Medication Guide.


INDICATIONS & USAGE SECTION
1 INDICATIONS AND USAGE
Lanthanum carbonate chewable tablet is a phosphate binder indicated to reduce
serum phosphate in patients with end-stage renal disease (ESRD).
Management of elevated serum phosphorus levels in patients with ESRD usually
includes all of the following: reduction in dietary intake of phosphate,
removal of phosphate by dialysis, and reduction of intestinal phosphate
absorption with phosphate binders.
- Lanthanum carbonate chewable tablet is a phosphate binder indicated to reduce serum phosphate in patients with end-stage renal disease (ESRD). (1)
CONTRAINDICATIONS SECTION
4 CONTRAINDICATIONS
Contraindicated in bowel obstruction, including ileus and fecal impaction.
- Bowel obstruction, ileus, and fecal impaction. (4)
WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Gastrointestinal Adverse Effects
Serious cases of gastrointestinal obstruction, ileus, subileus, gastrointestinal perforation, and fecal impaction have been reported in patients taking lanthanum, some requiring surgery or hospitalization.
Risk factors for gastrointestinal obstruction and gastrointestinal perforation identified from post-marketing reports in patients taking lanthanum carbonate chewable tablets include abnormal gastrointestinal anatomy (e.g., diverticular disease, peritonitis, history of gastrointestinal surgery, gastrointestinal cancer, gastrointestinal ulceration), hypomotility disorders (e.g., constipation, ileus, subileus, diabetic gastroparesis), and the use of medications known to potentiate these effects. Some cases were reported in patients with no history of gastrointestinal disease.
Patients with acute peptic ulcer, ulcerative colitis, Crohn's disease or bowel obstruction were not included in lanthanum carbonate chewable tablets clinical studies [see Contraindications (4)].
Advise patients who are prescribed lanthanum carbonate chewable tablets to chew the tablet completely and not to swallow them whole. Serious gastrointestinal complications have been reported in association with unchewed or incompletely chewed tablets.
5.2 Diagnostic Tests
Lanthanum carbonate chewable tablets have radio-opaque properties and therefore may give the appearance typical of an imaging agent during abdominal X-ray procedures.Postmarketing reports of product residue have been reported during endoscopic imaging.
- Serious cases of gastrointestinal obstruction, ileus, subileus, gastrointestinal perforation,and fecal impaction. Risks include altered gastrointestinal anatomy, hypomotility disorders, and concomitant medications. Advise patients to chew or crush the tablet completely. (5.1)
- Lanthanum carbonate chewable tablets have radio-opaque properties and, therefore may give the appearance typical of an imaging agent during abdominal X-ray procedures. (5.2)
ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in other sections of the labeling:
- Gastrointestinal Adverse Effects [see Warnings and Precautions (5.1)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Overall, the safety profile of lanthanum carbonate chewable tablets have been studied in over 5,200 subjects in completed clinical trials. The most common adverse reactions for lanthanum carbonate chewable tablets were gastrointestinal events, such as nausea, vomiting, and abdominal pain and they generally abated over time with continued dosing.
In double-blind, placebo-controlled studies where a total of 180 and 95 patients with ESRD were randomized to lanthanum carbonate chewable tablets and placebo, respectively, for 4 to 6 weeks of treatment, the most common reactions that were more frequent (≥5% difference) in the lanthanum carbonate chewable tablets group were nausea, vomiting, and abdominal pain (Table 1).
Table 1: Adverse Reactions That Were More Common on Lanthanum Carbonate
Chewable*
** Tablets in Placebo-Controlled, Double-Blind Studies with Treatment Periods
of 4 to 6 Weeks**
| ||
|
Lanthanum Carbonate Chewable Tablets |
Placebo | |
|
Nausea |
11 |
5 |
|
Vomiting |
9 |
4 |
|
Abdominal pain |
5 |
0 |
In an open-label, long-term 2 year extension study in 93 patients who had transitioned from other studies, resulting in a total of up to 6 years treatment, mean baseline values and changes in transaminases were similar to those observed in the earlier comparative studies, with little change during treatment.
The safety of lanthanum carbonate chewable tablets was studied in two long- term, open-label clinical trials, which included 1,215 patients treated with lanthanum carbonate chewable tablets and 944 with alternative therapy. Fourteen percent (14%) of patients treated with lanthanum carbonate chewable tablets discontinued treatment due to adverse events. Gastrointestinal adverse reactions, such as nausea, diarrhea, and vomiting were the most common types of event leading to discontinuation.
In pooled active comparator controlled clinical trials, hypocalcemia was noted with an incidence of approximately 5% in both lanthanum and active comparator groups. A nonclinical study and a phase 1 study have shown reduced absorption of calcium in the intestine with lanthanum carbonate treatment.
In a crossover study in 72 healthy individuals comparing lanthanum chewable tablets to lanthanum oral powder, gastrointestinal adverse reactions such as nausea, diarrhea, and vomiting were more common for the oral powder formulation (18%) than for the chewable tablets (7%).
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of lanthanum carbonate chewable tablets. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Cases of constipation, intestinal perforation, intestinal obstruction, ileus, subileus, dyspepsia, allergic skin reactions, hypophosphatemia, and tooth injury while chewing the tablet have been reported.
- In controlled trials, the most common adverse reactions that were more frequent (≥ 5% difference vs. placebo) in lanthanum carbonate chewable tablets were nausea, vomiting, and abdominal pain. (6.1)
- The following adverse reactions have been identified during post-approval use of lanthanum carbonate chewable tablets: constipation, dyspepsia, allergic skin reactions, and tooth injury while chewing the tablet. (6.2)
To report SUSPECTED ADVERSE REACTIONS, contact Lupin Pharmaceuticals, Inc. at 1-800-399-2561 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
7.1 Drugs Binding to Antacids
There is a potential for lanthanum carbonate chewable tablets to interact with compounds which bind to cationic antacids (i.e., aluminum-, magnesium-, or calcium-based); therefore, do not administer such compounds within 2 hours of dosing with lanthanum carbonate chewable tablets. Examples of relevant classes of compounds where antacids have been demonstrated to reduce bioavailability include antibiotics (such as quinolones, ampicillin, and tetracyclines), thyroid hormones, ACE-inhibitors, statin lipid regulators, and anti-malarials.
7.2 Quinolone Antibiotics
Co-administration of lanthanum carbonate chewable tablets with quinolone antibiotics may reduce the extent of their absorption. The bioavailability of oral ciprofloxacin was decreased by approximately 50% when taken with lanthanum carbonate chewable tablets in a single-dose study in healthy volunteers. Administer oral quinolone antibiotics at least 1 hour before or 4 hours after lanthanum carbonate chewable tablets. When oral quinolones are given for short courses, consider eliminating the doses of lanthanum carbonate chewable tablets that would normally be scheduled near the time of quinolone intake to improve quinolone absorption [see Clinical Pharmacology (12.3)].
7.3 Levothyroxine
The bioavailability of levothyroxine was decreased by approximately 40% when taken together with lanthanum carbonate chewable tablets. Administer thyroid hormone replacement therapy at least 2 hours before or 2 hours after dosing with lanthanum carbonate chewable tablets and monitor thyroid stimulating hormone (TSH) levels [see Clinical Pharmacology(12.3)].
7.4 Use with Other Oral Medications
There are no empirical data on avoiding drug interactions between lanthanum carbonate chewable tablets and most concomitant oral drugs. For oral medications where a reduction in the bioavailability of that medication would have a clinically significant effect on its safety or efficacy, consider separation of the timing of the administration of the two drugs. The duration of separation depends upon the absorption characteristics of the medication concomitantly administered, such as the time to reach peak systemic levels and whether the drug is an immediate-release or an extended-release product. Consider monitoring clinical responses or blood levels of concomitant medications that have a narrow therapeutic range.
- There is a potential for lanthanum carbonate chewable tablets to interact with compounds that bind to cationic antacids (i.e., aluminum-, magnesium-, or calcium-based); therefore, do not take such compounds within 2 hours of dosing with lanthanum carbonate chewable tablets. (7.1)
- Oral quinolone antibiotics must be taken at least 1 hour before or 4 hours after lanthanum carbonate chewable tablets. (7.2)
- Do not take thyroid hormone replacement therapy within 2 hours of dosing with lanthanum carbonate chewable tablets. Monitoring of TSH levels is recommended in patients receiving both medicinal agents. (7.3)
- For oral medications where a reduction in the bioavailability of that medication would have a clinically significant effect on its safety or efficacy, consider separation of the timing if the administration of the two drugs. (7.4)
USE IN SPECIFIC POPULATIONS SECTION
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Available data from case reports with use of lanthanum carbonate chewable
tablets in pregnant women are insufficient to identify a drug-associated risk
of major birth defects, miscarriage, or adverse maternal or fetal outcomes. In
animal reproduction studies, oral administration of lanthanum carbonate to
pregnant rats and rabbits during organogenesis at doses 3 and 2.5 times,
respectively, the maximum recommended human dose (MRHD), resulted in no
adverse developmental effects. In rabbits, lanthanum carbonate doses 5 times
the MRHD was associated with maternal toxicity and resulted in increased post-
implantation loss, reduced fetal weights, and delayed fetal ossification (see
Data). Deposition of lanthanum into developing bone, including growth plate,
was observed in juvenile animals in long-term animal studies with lanthanum
carbonate [see Use in Specific Populations (8.4)]. Use a non-lanthanum
containing phosphate binder in a pregnant woman.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defect and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
In pregnant rats, oral administration of lanthanum carbonate at doses as high
as 2,000 mg/kg/day during organogenesis resulted in no evidence of harm to the
fetus. The MRHD for lanthanum carbonate chewable tablet is 5,725 mg,
representing a dose of 95.4 mg/kg, or 3,530 mg/m2 for a 60-kg patient. The
2,000-mg/kg/day dose in the rat is equivalent to 12,000 mg/m2, 3 times the
MRHD. In pregnant rabbits, oral administration of lanthanum carbonate at 1,500
mg/kg/day (18,000 mg/m2; 5 times the daily MRHD) during organogenesis was
associated with increased postimplantation loss, reduced fetal weights, and
delayed fetal ossification. No effects on the pregnant rabbits or fetuses were
observed at 750 mg/kg/day (9,000 mg/m2; 2.5 times the MRHD).
In a pre-and postnatal development study in the rat, pregnant rats were dosed at up to 2,000 mg/kg/day (12,000 mg/m2/day; equivalent to 3 times the MRHD) from day 6 of pregnancy through 20 days postpartum (including lactation). At 2,000 mg/kg/day, no maternal toxicity was observed, nor were any changes seen with respect to gestational length or delivery; however, piloerection/pallor, delayed eye opening, decreased body weight, and delayed sexual development were observed in the offspring at 2,000 mg/kg/day. At 200 and 600 mg/kg/day (equivalent to 0.3 and 1 time the MRHD, respectively), slight delays in sexual development (delayed vaginal opening) were observed in the female offspring [see Nonclinical Toxicology (13.2)].
8.2 Lactation
Risk Summary
There are no data on the presence of lanthanum carbonate from lanthanum
carbonate chewable tablets in human milk, the effects on the breastfed infant,
or the effects on milk production. Deposition of lanthanum into developing
bone, including growth plate, was observed in juvenile animals in long-term
animal studies with lanthanum carbonate [see Use in Specific Populations (8.4)].Use a non-lanthanum containing phosphate binder in a lactating woman.
8.4 Pediatric Use
The safety and efficacy of lanthanum carbonate chewable tablets in pediatric patients have not been established. While growth abnormalities were not identified in long-term animal studies, lanthanum was deposited into developing bone including growth plate. The consequences of such deposition in developing bone, in pediatric patients are unknown; therefore, the use of lanthanum carbonate chewable tablets in this population is not recommended.
8.5 Geriatric Use
Of the total number of patients in clinical studies of lanthanum carbonate chewable tablets, 32% (538) were ≥65 years of age, while 9.3% (159) were ≥75 years of age. No overall differences in safety or effectiveness were observed between patients ≥65 years of age and younger patients.
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
Divide the total daily dose of lanthanum carbonate chewable tablets and take with or immediately after meals. The recommended initial total daily dose of lanthanum carbonate chewable tablets is 1,500 mg. Titrate the dose every 2 to 3 weeks until an acceptable serum phosphate level is reached. Monitor serum phosphate levels as needed during dose titration and on a regular basis thereafter.
Lanthanum carbonate chewable tablets have the potential to bind other orally administered drugs; consider separating the administration of other oral medications [see Drug Interactions (7)].
In clinical studies patients with ESRD, lanthanum carbonate chewable tablets doses up to 4,500 mg were evaluated. Most patients required a total daily dose between 1,500 mg and 3,000 mg to reduce plasma phosphate levels to less than 6.0 mg/dL. Doses were generally titrated in increments of 750 mg/day.
Information for lanthanum carbonate chewable tablets
Chew or crush lanthanum carbonate chewable tablets completely before
swallowing. Do not swallow intact lanthanum carbonate chewable tablets.
Consider using the oral powder formulation in patients with poor dentition or who have difficulty chewing tablets.
- The recommended initial total daily dose of lanthanum carbonate chewable tablets is 1,500 mg in divided doses. Titrate every 2 to 3 weeks based on serum phosphate level. (2)
- Take lanthanum carbonate chewable tablets with or immediately after meals. (2)
- Lanthanum carbonate chewable tablets: Chew or crush tablet completely before swallowing. (2)
- Consider powder formulation in patients with poor dentition or who have difficulty chewing tablets (2)
DOSAGE FORMS & STRENGTHS SECTION
3 DOSAGE FORMS AND STRENGTHS
Lanthanum Carbonate Chewable Tablets: 500 mg, 750 mg, and 1,000 mg.
- Lanthanum Carbonate Chewable Tablets: 500 mg, 750 mg and 1,000 mg. (3)
OVERDOSAGE SECTION
10 OVERDOSAGE
The symptoms associated with overdose are adverse reactions such as headache, nausea and vomiting. In clinical trials in healthy adults, gastrointestinal (GI) symptoms were reported with daily doses up to 6,000 mg/day of lanthanum carbonate administered with food. Given the topical activity of lanthanum in the gut, and the excretion of the majority of the dose in feces, supportive therapy is recommended for overdosage. Lanthanum carbonate was not acutely toxic in animals by the oral route. No deaths and no adverse effects occurred in mice, rats, or dogs after single oral doses of 2,000 mg/kg (1.7, 3.4, and 11.3 times the MRHD, respectively, on a mg/m2 basis).
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 Lanthanum carbonate chewable tablets
Lanthanum carbonate chewable tablets are supplied in three dosage strengths as lanthanum for oral administration: 500 mg tablets, 750 mg tablets, and 1000 mg tablets.
Lanthanum carbonate chewable tablets, 500 mg (as lanthanum) are white to off white round shaped tablets debossed ‘NAT’ on one side and ‘500’ on other side, supplied in patient pack (2 bottles of 45 chewable tablets each NDC 68180-819-52) NDC 68180-819-42.
Lanthanum carbonate chewable tablets, 750 mg (as lanthanum) are white to off white round shaped tablets debossed ‘NAT’ on one side and ‘750’ on other side, supplied in patient pack (6 bottles of 15 chewable tablets each NDC 68180-820-53) NDC 68180-820-46.
Lanthanum carbonate chewable tablets, 1000 mg (as lanthanum) are white to off white round shaped tablets debossed ‘NAT’ on one side and ‘1000’ on other side, supplied in patient pack (9 bottles of 10 chewable tablets each NDC 68180-821-10) NDC 68180-821-47.
Storage and Handling
Store lanthanum carbonate chewable tablets at 25ºC (77ºF): excursions
permitted to 15º to 30˚C (59º to 86ºF).
[See USP controlled room temperature].
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
- Advise the patient to read the FDA-approved patient labeling (Medication Guide).
- Advise patients to take lanthanum carbonate chewable tablets with or immediately after meals [see Dosage and Administration (2)].
- Instruct patients on concomitant medications that should be dosed apart from lanthanum carbonate chewable tablets [see Drug Interactions (7)].
- Instruct patients who are prescribed lanthanum carbonate chewable tablets to chew or crush tablets completely before swallowing**.** Emphasize that lanthanum carbonate chewable tablets should not be swallowed intact. Consider crushing lanthanum carbonate chewable tablets completely or prescribing the oral powder formulation for patients with poor dentition or who have difficulty chewing tablets [see Dosage and Administration (2)].
- Advise patients who are taking an oral medication where a reduction in the bioavailability of that medication would have a clinically significant effect on its safety or efficacy to separate the dosing of lanthanum carbonate chewable tablets from the dosing of the affected drug by several hours [see Drug Interactions (7)].
- Advise patients to notify their physician that they are taking lanthanum carbonate chewable tablets prior to an abdominal X-ray or if they have a history of gastrointestinal disease [see Warnings and Precautions (5.1, 5.2)].
Manufactured by:
NATCO PHARMA LIMITED
India.
Distributed by:
Lupin Pharmaceuticals, Inc.
Baltimore, Maryland 21202
United States
** Rev: Dec 2023**
MEDICATION GUIDE
** Lanthanum Carbonate (LAN-tha-num KAR-bo-nate) Chewable Tablets**
Read this Medication Guide before you start taking lanthanum carbonate
chewable tablets and each time you get a refill. There may be new information.
This information does not take the place of talking to your healthcare
provider about your medical condition or treatment.
What is the most important information I should know about lanthanum carbonate chewable tablets?
****Lanthanum carbonate chewable tablets may cause a bowel blockage ,a hole in the bowel, or severe constipation, which can be serious, and sometimes lead to surgery or treatment in a hospital.
- You may have a higher risk of bowel blockage, a hole in the bowel, or severe constipation if you take lanthanum carbonate chewable tablets and have:
- a history of surgery, ulcers or cancer in the stomach or bowel
- a history of bowel blockage, or problems resulting in a decreased movement of food through your stomach and bowel (e.g., feeling full quickly after eating or constipation)
- an infection or inflammation of the stomach/bowel (peritonitis)
Do not swallow lanthanum carbonate chewable tablets whole. Chew tablets completely before swallowing. If you cannot chew tablets completely, you may crush the tablets thoroughly before swallowing or discuss the oral powder formulation with your health care provider.
What is lanthanum carbonate chewable tablet?
Lanthanum carbonate chewable tablet is a prescription medicine used in people with end-stage renal disease (ESRD) to lower the amount of phosphate in the blood.
Who should not take lanthanum carbonate chewable tablets?
****Do not take lanthanum carbonate chewable tablets if you:
- have blocked bowels
- have severe constipation
Lanthanum carbonate chewable tablets have not been studied in children and adolescents under 18 years of age.
** What should I tell my healthcare provider before taking lanthanum carbonate chewable tablets?**
Lanthanum carbonate chewable tablets may not be right for you. Before starting lanthanum carbonate chewable tablets, tell your healthcare provider if you:
-
have a history of surgery, ulcers or cancer in the stomach or bowel
-
have a history of a bowel blockage, constipation, or problems resulting in a decreased movement of food through your stomach and bowel especially if you also have diabetes
-
have ulcerative colitis, Crohn’s disease or an infection or inflammation of the stomach/bowel (peritonitis)
-
plan to have an X-ray of your stomach (abdomen)
-
have any other medical conditions
-
are pregnant, plan to become pregnant, or plan to breastfeed.Tell your healthcare provider about all of the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements.
-
Especially tell your healthcare provider if you take:
-
antacids
-
antibiotics
-
thyroid medicine
Know the medicines you take. Keep a list of them and show it to your healthcare provider and pharmacist when you get a new medicine.
How should I take lanthanum carbonate chewable tablets?
-
Take lanthanum carbonate chewable tablets exactly as prescribed by your healthcare provider
-
Your healthcare provider will tell you how much lanthanum carbonate chewable tablets to take
-
Your healthcare provider may change your dose if needed
*Chewable tablets - Do not swallow tablets whole. Chew tablets completely before swallowing. If you cannot chew tablets completely, or if you have tooth disease, you may crush the tablets thoroughly before swallowing or discuss the oral powder formulation with your healthcare provider.
-
Take lanthanum carbonate chewable tablets with or right after meals
-
If you take an antacid medicine, take the antacid 2 hours before or 2 hours after you take lanthanum carbonate chewable tablets
-
If you take medicine for your thyroid (levothyroxine), take the thyroid medicine 2 hours before or 2 hours after you take lanthanum carbonate chewable tablets
-
If you take an antibiotic medicine, take the antibiotic 1 hour before or 4 hours after you take lanthanum carbonate chewable tablets
What are possible or reasonably likely side effects of lanthanum carbonate chewable tablets?
See** “What is the most important information I should know about lanthanum carbonate chewable tablets?”**
The most common side effects of lanthanum carbonate chewable tablets include:
-
nausea
-
vomiting
-
diarrhea
-
stomach pain
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the side effects of lanthanum carbonate chewable tablets. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store lanthanum carbonate chewable tablets?
- Store lanthanum carbonate chewable tablets between 59°F to 86°F (15°C to 30°C).
Keep lanthanum carbonate chewable tablets and all medicines out of the reach of children.
** General information about lanthanum carbonate chewable tablets**
****Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use lanthanum carbonate chewable tablets for a condition for which it was not prescribed. Do not give lanthanum carbonate chewable tablets to other people, even if they have the same condition. It may harm them.
This Medication Guide summarizes the most important information about
lanthanum carbonate chewable tablets. If you would like more information, talk
with your healthcare provider. You can ask your pharmacist or healthcare
provider for information about lanthanum carbonate chewable tablets that is
written for healthcare professionals.
For more information go to www.lupinpharmaceuticals.com or call
1-800-399-2561.
What are the ingredients in lanthanum carbonate chewable tablets?
Active ingredient: lanthanum carbonate
Inactive ingredients: colloidal silicon dioxide, dextrates, hydroxy propyl cellulose, magnesium stearate and talc.
This Medication Guide has been approved by the US Food and Drug Administration.
Manufactured by:
NATCO PHARMA LIMITED
****India.
Distributed by:
Lupin Pharmaceuticals, Inc.
****Baltimore, Maryland 21202
United States
Rev: Jan 2024
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Lanthanum carbonate chewable tablet is a phosphate binder that reduces absorption of phosphate by forming insoluble lanthanum phosphate complexes that pass through the GI tract unabsorbed. Both serum phosphate and calcium phosphate product are reduced as a consequence of the reduced dietary phosphate absorption.
12.2 Pharmacodynamics
In vitro studies have shown that lanthanum binds phosphate in the physiologically relevant pH range of 3 to 7. In simulated gastric fluid, lanthanum binds approximately 97% of the available phosphate at pH 3 to 5 and 67% at pH 7, when lanthanum is present in a two-fold molar excess to phosphate. Bile acids have not been shown to affect the phosphate binding affinity of lanthanum. In order to bind dietary phosphate, lanthanum carbonate chewable tablets must be administered with or immediately after meals.
In five phase I pharmacodynamic studies comparing the reduction from baseline of urinary phosphorus excretion in healthy volunteers (N=143 taking lanthanum carbonate), it was shown that the mean intestinal phosphate binding capacity of lanthanum ranged from 235 to 468 mg phosphorus/day when lanthanum was administered at a dose of 3 g per day with food. By comparison, in one study with an untreated control group (n=10) and another study with a placebo group (n=3), the corresponding mean changes from baseline were 3 mg phosphorus/day and 87 mg phosphorus/day, respectively.
In healthy subjects, lanthanum carbonate oral powder and lanthanum carbonate chewable tablets produce similar effect on urinary phosphate excretion.
12.3 Pharmacokinetics
Absorption and Distribution -Following single- or multiple-dose oral administration of lanthanum carbonate chewable tablets to healthy subjects, the concentration of lanthanum in plasma was very low (bioavailability <0.002%). Following oral administration in patients, the mean lanthanum Cmax was 1.0 ng/mL. During long-term administration (52 weeks) in patients with ESRD, the mean lanthanum concentration in plasma was approximately 0.6 ng/mL. There was a minimal increase in plasma lanthanum concentrations with increasing doses within the therapeutic dose range. The timing of food intake relative to lanthanum administration (during and 30 minutes after food intake) has a negligible effect on the systemic level of lanthanum.
Systemic exposure to lanthanum was approximately 30% higher following administration of lanthanum carbonate oral powder when compared to lanthanum carbonate chewable tablets. However, systemic exposure to lanthanum from both formulations in this study was within the range seen in previous pharmacokinetics studies of chewable tablets in healthy individuals.
In vitro, lanthanum is highly bound (>99%) to human plasma proteins, including human serum albumin, α1-acid glycoprotein, and transferrin. Binding to erythrocytes in vivo is negligible in rats.
In animal studies, lanthanum concentrations in several tissues, particularly gastrointestinal tract, mesenteric lymph nodes, bone, and liver, increased over time to levels several orders of magnitude higher than those in plasma. The level of lanthanum in the liver was higher in renally impaired rats due to higher intestinal absorption. Lanthanum was found in the lysosomes and the biliary canal consistent with transcellular transport. Steady state tissue concentrations in bone and liver were achieved in dogs between 4 and 26 weeks. Relatively high levels of lanthanum remained in these tissues for longer than 6 months after cessation of dosing in dogs. There is no evidence from animal studies that lanthanum crosses the blood-brain barrier.
In 105 bone biopsies from patients treated with lanthanum carbonate chewable tablets for up to 4.5 years, rising levels of lanthanum were noted over time. Cases of lanthanum deposition in gastrointestinal mucosa, mainly after long term use, have been reported. The clinical significance of this is yet unknown. Estimates of elimination half-life from bone ranged from 2.0 to 3.6 years. Steady state bone concentrations were not reached during the period studied.
Metabolism and Elimination - Lanthanum is not metabolized. Lanthanum was cleared from plasma of patients undergoing dialysis with an elimination half- life of 53 hours following discontinuation of therapy.
No information is available regarding the mass balance of lanthanum in humans after oral administration. In rats and dogs, the mean recovery of lanthanum after an oral dose was about 99% and 94%, respectively, and was essentially all from feces. Biliary excretion is the predominant route of elimination for circulating lanthanum in rats. In healthy volunteers administered intravenous (IV) lanthanum as the soluble chloride salt (120 mcg), renal clearance was less than 2% of total plasma clearance.
Drug Interactions
Lanthanum carbonate chewable tablets have a low potential for systemic drug-
drug interactions because of the very low bioavailability of lanthanum and
because it is not a substrate or inhibitor of major cytochrome P450 enzyme
groups involved in drug metabolism (CYP1A2, CYP2C9/10, CYP2C19, CYP2D6, and
CYP3A4/5).
Lanthanum carbonate chewable tablets do not alter gastric pH; therefore, lanthanum carbonate chewable tablets drug interactions based on altered gastric pH are not expected.
In an in vitro investigation, lanthanum did not form insoluble complexes when mixed in simulated gastric fluid with warfarin, digoxin, furosemide, phenytoin, metoprolol, and enalapril. Clinical studies have shown that lanthanum carbonate chewable tablets (three doses of 1,000 mg on the day prior to exposure and one dose of 1,000 mg on the day of co-administration) administered 30 minutes earlier did not alter the pharmacokinetics of oral warfarin (10 mg), digoxin (0.5 mg), or metoprolol (100 mg). Potential pharmacodynamic interactions between lanthanum and these drugs (e.g., bleeding time or prothrombin time) were not evaluated. None of the drug interaction studies were done with the maximum recommended therapeutic dose of lanthanum carbonate. No drug interaction studies assessed the effects of drugs on phosphate binding by lanthanum carbonate.
Ciprofloxacin
In a randomized, two–way crossover study in healthy volunteers examining the interaction potential of a single oral dose of ciprofloxacin (750 mg) alone and with lanthanum carbonate (1 g three times a day), the maximum plasma concentration of ciprofloxacin was reduced by 56% and the area under the ciprofloxacin plasma concentration-time curve was reduced by 54%. The 24-hour urinary recovery of ciprofloxacin was reduced 52% by lanthanum carbonate chewable tablets [see Drug Interactions (7.2)].
Levothyroxine
In a single-dose crossover study of levothyroxine (1 mg) with or without simultaneous administration of a single dose of lanthanum carbonate chewable tablets (500 mg) in six euthyroid normal healthy volunteers, the area under the serum T4 concentration-time curve was decreased by 40% [see Drug Interactions (7.3)].
Fat-Soluble Vitamins
Lanthanum carbonate chewable tablet appears not to affect the availability of fat-soluble vitamins (A, D, E, and K) or other nutrients [see Clinical Studies (14.2)].
****Citrate
Citrate did not increase the absorption of lanthanum.
DESCRIPTION SECTION
11 DESCRIPTION
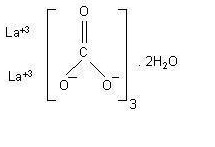
Lanthanum carbonate chewable tablet contains lanthanum carbonate dihydrate with molecular formula La2(CO3)3 .2H2O and molecular weight 457.8 (anhydrous mass). Lanthanum carbonate is described as white to off-white powder. Lanthanum carbonate is soluble in dilute hydrochloric acid and practically insoluble in water.
Each lanthanum carbonate chewable tablets, white to off-white, chewable tablet contains lanthanum carbonate dihydrate equivalent to 500, 750 or 1,000 mg of elemental lanthanum and the following inactive ingredients: colloidal silicon dioxide, dextrates, hydroxy propyl cellulose, magnesium stearate and talc.
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Oral administration of lanthanum carbonate to rats for up to 104 weeks, at doses up to 1,500 mg of the salt per kg/day (2.5 times the MRHD of 5,725 mg, on a mg/m2 basis, assuming a 60-kg patient) revealed no evidence of carcinogenic potential. In the mouse, oral administration of lanthanum carbonate for up to 99 weeks, at a dose of 1,500 mg/kg/day (1.3 times the MRHD) was associated with an increased incidence of glandular stomach adenomas in male mice.
Lanthanum carbonate tested negative for mutagenic activity in an in vitro Ames assay using Salmonella typhimurium and Escherichia coli strains and in vitro HGPRT gene mutation and chromosomal aberration assays in Chinese hamster ovary cells. Lanthanum carbonate also tested negative in an oral mouse micronucleus assay at doses up to 2,000 mg/kg (1.7 times the MRHD), and in micronucleus and unscheduled DNA synthesis assays in rats given IV lanthanum chloride at doses up to 0.1 mg/kg, a dose that produced plasma lanthanum concentrations greater than 2,000 times the peak human plasma concentration.
Lanthanum carbonate, at doses up to 2,000 mg/kg/day (3.4 times the MRHD), did not affect fertility or mating performance of male or female rats.
13.2 Animal Toxicology and/or Pharmacology
In pregnant rats, oral administration of lanthanum carbonate at doses as high as 2,000 mg/kg/day (3.4 times the MRHD) resulted in no evidence of harm to the fetus. In pregnant rabbits, oral administration of lanthanum carbonate at 1,500 mg/kg/day (5 times the MRHD) was associated with a reduction in maternal body weight gain and food consumption, increased post-implantation loss, reduced fetal weights, and delayed fetal ossification. No effects on pregnant rabbits or fetuses were observed at 750 mg/kg/day (2.5 times the MRHD). Lanthanum carbonate administered to rats from implantation through lactation at 2,000 mg/kg/day (3.4 times the MRHD) caused delayed eye opening, reduction in body weight gain, and delayed sexual development (preputial separation and vaginal opening) of the offspring. At 200 and 600 mg/kg/day (equivalent to 0.3 and 1 time the MRHD, respectively), slight delays in vaginal opening were observed in the female offspring.
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
The effectiveness of lanthanum carbonate chewable tablets in reducing serum phosphorus in patients with ESRD was demonstrated in one short-term, placebo- controlled, double-blind dose-ranging study, two placebo-controlled randomized withdrawal studies and two long-term, active-controlled, open-label studies in patients undergoing either hemodialysis and peritoneal dialysis.
14.1 Double-Blind Placebo-Controlled Studies
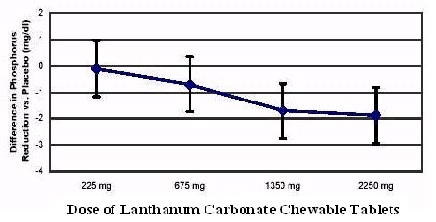
One hundred and forty-four patients with chronic renal failure undergoing hemodialysis and with elevated phosphate levels were randomized to double- blind treatment at a fixed dose of lanthanum carbonate of 225 mg (n=27), 675 mg (n=29), 1,350 mg (n=30), or 2,250 mg (n=26) or placebo (n=32) in divided doses with meals. Fifty-five percent of subjects were male, 71% black, 25% white, and 4% of other races. The mean age was 56 years and the duration of dialysis ranged from 0.5 to 15.3 years. Steady-state effects were achieved after two weeks. The effect after six weeks of treatment is shown in Figure 1.
Figure 1: Difference in Phosphate Reduction in the Lanthanum Carbonate
Chewable Tablets** and Placebo Group in a 6-Week, Dose-Ranging, Double-Blind
Study in ESRD Patients (with 95% Confidence Intervals)**
****
One-hundred and eighty-five patients with ESRD undergoing either hemodialysis (n=146) or peritoneal dialysis (n=39) were enrolled in two placebo- controlled,randomized withdrawal studies. Sixty-four percent of subjects were male, 28% black, 62% white, and 10% of other races. The mean age was 58.4 years and the duration of dialysis ranged from 0.2 to 21.4 years. After titration of lanthanum carbonate to achieve a phosphate level between 4.0 and 5.6 mg/dL in one study (doses up to 2,250 mg/day) or <5.9 mg/dL in the second study (doses up to 3,000 mg/day) and maintenance through 6 weeks, patients were randomized to lanthanum or placebo. During the placebo-controlled, randomized withdrawal phase (four weeks), the phosphorus concentration rose in the placebo group by 1.7 mg/dL in one study and 1.9 mg/dL in the other study relative to patients who remained on lanthanum carbonate therapy.
14.2 Open-Label Active-Controlled Studies
Two long-term open-label studies were conducted, involving a total of 2,028 patients with ESRD undergoing hemodialysis. Patients were randomized to receive lanthanum carbonate chewable tablets or alternative phosphate binders for up to six months in one study and two years in the other. The daily lanthanum carbonate chewable tablets dose, divided and taken with meals, ranged from 375 mg to 3,000 mg. Doses were titrated to reduce serum phosphate levels to a target level. The daily doses of the alternative therapy were based on current prescribing information or those commonly utilized. Both treatment groups had similar reductions in serum phosphate of about 1.8 mg/dL. Maintenance of reduction was observed for up to three years in patients treated with lanthanum carbonate chewable tablets in long-term, open-label extensions.
No effects of lanthanum carbonate chewable tablets on serum levels of 25-dihydroxy vitamin D3, vitamin A, vitamin B12, vitamin E, and vitamin K were observed in patients who were monitored for 6 months.
Paired bone biopsies (at baseline and at one or two years) in 69 patients randomized to either lanthanum carbonate chewable tablets or calcium carbonate in one study and 99 patients randomized to either lanthanum carbonate chewable tablets or alternative therapy in a second study showed no differences in the development of mineralization defects between the groups.
Vital status was known for over 2,000 patients, 97% of those participating in the clinical program during and after receiving treatment. The adjusted yearly mortality rate (rate/years of observation) for patients treated with lanthanum carbonate chewable tablets or alternative therapy was 6.6%.
