ABILIFY
These highlights do not include all the information needed to use ABILIFY safely and effectively. See full prescribing information for ABILIFY. ABILIFY (aripiprazole) Tablets, for oral use ABILIFY DISCMELT (aripiprazole) Orally Disintegrating Tablets ABILIFY (aripiprazole) Oral Solution ABILIFY (aripiprazole) Injection FOR INTRAMUSCULAR USE ONLY Initial U.S. Approval: 2002
c040bd1d-45b7-49f2-93ea-aed7220b30ac
HUMAN PRESCRIPTION DRUG LABEL
Nov 30, 2022
Otsuka America Pharmaceutical, Inc.
DUNS: 008314390
Products 10
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
ARIPIPRAZOLE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
ARIPIPRAZOLE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
ARIPIPRAZOLE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (4)
ARIPIPRAZOLE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
ARIPIPRAZOLE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (10)
ARIPIPRAZOLE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (14)
ARIPIPRAZOLE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (14)
ARIPIPRAZOLE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
ARIPIPRAZOLE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
ARIPIPRAZOLE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (8)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
30 Tablets (3 x 10) NDC 59148-641-23
ABILIFY DISCMELT®15 mg
(aripiprazole) Rx only
Orally Disintegrating Tablets
DISPENSE WITH MEDICATION GUIDE
Bristol-Myers Squibb
Otsuka America Pharmaceutical, Inc.
BOXED WARNING SECTION
WARNING: INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED
PSYCHOSIS and SUICIDAL THOUGHTS AND BEHAVIORS WITH ANTIDEPRESSANT DRUGS
See full prescribing information for complete boxed warning.
*Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. ABILIFY is not approved for the treatment of patients with dementia-related psychosis. (5.1) *Increased risk of suicidal thinking and behavior in children, adolescents, and young adults taking antidepressants. Monitor for worsening and emergence of suicidal thoughts and behaviors. (5.3)
INDICATIONS & USAGE SECTION
1 INDICATIONS AND USAGE
ABILIFY (aripiprazole) Oral Tablets, Orally-Disintegrating Tablets, and Oral Solution are indicated for the treatment of:
- Schizophrenia
- Acute Treatment of Manic and Mixed Episodes associated with Bipolar I Disorder
- Adjunctive Treatment of Major Depressive Disorder
- Irritability Associated with Autistic Disorder
- Treatment of Tourette's Disorder
ABILIFY Injection is indicated for the treatment of:
- Agitation associated with schizophrenia or bipolar mania
ABILIFY is an atypical antipsychotic. The oral formulations are indicated for:
- Schizophrenia (14.1)
- Acute Treatment of Manic and Mixed Episodes associated with Bipolar I (14.2)
- Adjunctive Treatment of Major Depressive Disorder (14.3)
- Irritability Associated with Autistic Disorder (14.4)
- Treatment of Tourette's disorder (14.5)
The injection is indicated for:
- Agitation associated with schizophrenia or bipolar mania (14.6)
CONTRAINDICATIONS SECTION
4 CONTRAINDICATIONS
ABILIFY is contraindicated in patients with a history of a hypersensitivity reaction to aripiprazole. Reactions have ranged from pruritus/urticaria to anaphylaxis [see Adverse Reactions (6.2)].
- Known hypersensitivity to ABILIFY (4)
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
7.1 Drugs Having Clinically Important Interactions with ABILIFY
Table 25: Clinically Important Drug Interactions with ABILIFY:|
Concomitant Drug Name or Drug Class |
Clinical Rationale |
Clinical Recommendation |
|---|---|---|
|
Strong CYP3A4 Inhibitors (e.g., itraconazole, clarithromycin) or strong CYP2D6 inhibitors (e.g., quinidine, fluoxetine, paroxetine) |
The concomitant use of ABILIFY with strong CYP 3A4 or CYP2D6 inhibitors increased the exposure of aripiprazole compared to the use of ABILIFY alone [see Clinical Pharmacology (12.3)]. |
With concomitant use of ABILIFY with a strong CYP3A4 inhibitor or CYP2D6 inhibitor, reduce the ABILIFY dosage [see Dosage and Administration (2.7)]. |
|
Strong CYP3A4 Inducers (e.g., carbamazepine, rifampin) |
The concomitant use of ABILIFY and carbamazepine decreased the exposure of aripiprazole compared to the use of ABILIFY alone [see Clinical Pharmacology (12.3)]. |
With concomitant use of ABILIFY with a strong CYP3A4 inducer, consider increasing the ABILIFY dosage [see Dosage and Administration (2.7)]. |
|
Antihypertensive Drugs |
Due to its alpha adrenergic antagonism, aripiprazole has the potential to enhance the effect of certain antihypertensive agents. |
Monitor blood pressure and adjust dose accordingly [see Warnings and Precautions (5.8)]. |
|
Benzodiazepines (e.g., lorazepam) |
The intensity of sedation was greater with the combination of oral aripiprazole and lorazepam as compared to that observed with aripiprazole alone. The orthostatic hypotension observed was greater with the combination as compared to that observed with lorazepam alone [see Warnings and Precautions (5.8)]. |
Monitor sedation and blood pressure. Adjust dose accordingly. |
7.2 Drugs Having No Clinically Important Interactions with ABILIFY
Based on pharmacokinetic studies, no dosage adjustment of ABILIFY is required when administered concomitantly with famotidine, valproate, lithium, lorazepam.
In addition, no dosage adjustment is necessary for substrates of CYP2D6 (e.g., dextromethorphan, fluoxetine, paroxetine, or venlafaxine), CYP2C9 (e.g., warfarin), CYP2C19 (e.g., omeprazole, warfarin, escitalopram), or CYP3A4 (e.g., dextromethorphan) when co-administered with ABILIFY. Additionally, no dosage adjustment is necessary for valproate, lithium, lamotrigine, lorazepam, or sertraline when co-administered with ABILIFY [see Clinical Pharmacology (12.3)].
Dosage adjustment due to drug interactions (7.1):
|
Factors |
Dosage Adjustments for ABILIFY |
|---|---|
|
Known CYP2D6 Poor Metabolizers |
Administer half of usual dose |
|
Known CYP2D6 Poor Metabolizers and strong CYP3A4 inhibitors |
Administer a quarter of usual dose |
|
Strong CYP2D6or CYP3A4 inhibitors |
Administer half of usual dose |
|
Strong CYP2D6and CYP3A4 inhibitors |
Administer a quarter of usual dose |
|
Strong CYP3A4 inducers |
Double usual dose over 1 to 2 weeks |
DOSAGE FORMS & STRENGTHS SECTION
3 DOSAGE FORMS AND STRENGTHS
ABILIFY® (aripiprazole) Tablets are available as described in 3.
Table 3: ABILIFY Tablet Presentations|
Tablet Strength |
Tablet Color/Shape |
Tablet Markings |
|---|---|---|
|
2 mg |
green |
"A-006" and "2" |
|
5 mg |
blue |
"A-007" and "5" |
|
10 mg |
pink |
"A-008" and "10" |
|
15 mg |
yellow |
"A-009" and "15" |
|
20 mg |
white |
"A-010" and "20" |
|
30 mg |
pink |
"A-011" and "30" |
ABILIFY DISCMELT® (aripiprazole) Orally Disintegrating Tablets are available as described in Table 4.
Table 4: ABILIFY DISCMELT Orally Disintegrating Tablet Presentations|
Tablet Strength |
Tablet Color/Shape |
Tablet Markings |
|---|---|---|
|
10 mg |
pink (with scattered specks) |
"A" and "640" |
|
15 mg |
yellow (with scattered specks) |
"A" and "641" |
ABILIFY® (aripiprazole) Oral Solution (1 mg/mL) is a clear, colorless to light-yellow solution, supplied in child-resistant bottles along with a calibrated oral dosing cup.
ABILIFY® (aripiprazole) Injection for Intramuscular Use is a clear, colorless solution available as a ready-to-use, 9.75 mg/1.3 mL (7.5 mg/mL) solution in clear, Type 1 glass vials.
- Tablets: 2 mg, 5 mg, 10 mg, 15 mg, 20 mg, and 30 mg (3)
- Orally Disintegrating Tablets: 10 mg and 15 mg (3)
- Oral Solution: 1 mg/mL (3)
- Injection: 9.75 mg/1.3 mL single-dose vial (3)
USE IN SPECIFIC POPULATIONS SECTION
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to atypical antipsychotics, including ABILIFY, during pregnancy. Healthcare providers are encouraged to register patients by contacting the National Pregnancy Registry for Atypical Antipsychotics at 1-866-961-2388 or visit http://womensmentalhealth.org/clinical-and-research- programs/pregnancyregistry/.
Risk Summary
Neonates exposed to antipsychotic drugs, including ABILIFY, during the third trimester of pregnancy are at risk for extrapyramidal and/or withdrawal symptoms following delivery (see Clinical Considerations). Overall available data from published epidemiologic studies of pregnant women exposed to aripiprazole have not established a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes (see Data). There are risks to the mother associated with untreated schizophrenia, bipolar I disorder, or major depressive disorder, and with exposure to antipsychotics, including ABILIFY, during pregnancy (see Clinical Considerations).
In animal reproduction studies, oral and intravenous aripiprazole administration during organogenesis in rats and/or rabbits at doses 10 and 19 times, respectively, the maximum recommended human dose (MRHD) of 30 mg/day based on mg/m2 body surface area, produced fetal death, decreased fetal weight, undescended testicles, delayed skeletal ossification, skeletal abnormalities, and diaphragmatic hernia. Oral and intravenous aripiprazole administration during the pre- and post-natal period in rats at doses 10 times the MRHD based on mg/m2 body surface area, produced prolonged gestation, stillbirths, decreased pup weight, and decreased pup survival (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Clinical Considerations
Disease-associated maternal and/or embryo/fetal risk
There is a risk to the mother from untreated schizophrenia or bipolar I disorder, including increased risk of relapse, hospitalization, and suicide. Schizophrenia and bipolar I disorder are associated with increased adverse perinatal outcomes, including preterm birth. It is not known if this is a direct result of the illness or other comorbid factors.
A prospective, longitudinal study followed 201 pregnant women with a history of major depressive disorder who were euthymic and taking antidepressants at the beginning of pregnancy. The women who discontinued antidepressants during pregnancy were more likely to experience a relapse of major depression than women who continued antidepressants. Consider the risk of untreated depression when discontinuing or changing treatment with antidepressant medication during pregnancy and postpartum.
Fetal/Neonatal Adverse Reactions
Extrapyramidal and/or withdrawal symptoms, including agitation, hypertonia, hypotonia, tremor, somnolence, respiratory distress, and feeding disorder have been reported in neonates who were exposed to antipsychotic drugs (including ABILIFY) during the third trimester of pregnancy. These symptoms have varied in severity. Monitor neonates for extrapyramidal and/or withdrawal symptoms and manage symptoms appropriately. Some neonates recovered within hours or days without specific treatment; others required prolonged hospitalization.
Data
Human Data
Published data from observational studies, birth registries, and case reports on the use of atypical antipsychotics during pregnancy do not report a clear association with antipsychotics and major birth defects. A retrospective study from a Medicaid database of 9258 women exposed to antipsychotics during pregnancy did not indicate an overall increased risk for major birth defects.
Animal Data
In animal studies, aripiprazole demonstrated developmental toxicity, including possible teratogenic effects in rats and rabbits.
In pregnant rats treated orally with aripiprazole during organogenesis at doses of 3, 10, and 30 mg/kg/day, which are approximately 1, 3 and 10 times the MRHD of 30 mg/day based on mg/m2 body surface area, a slight prolongation of gestation and delay in fetal development, as evidenced by decreased fetal weight and undescended testes, were observed at 10 times the MRHD. Delayed skeletal ossification was observed at 3 and 10 times the MRHD. Delivered offspring had increased incidences of hepatodiaphragmatic nodules and diaphragmatic hernia were observed at 10 times the MRHD (the other dose groups were not examined for these findings). Postnatally, delayed vaginal opening was seen at 3 and 10 times the MRHD. Impaired reproductive performance (decreased fertility rate, corpora lutea, implants, live fetuses, and increased post-implantation loss, likely mediated through effects on female offspring) were observed at 10 times the MRHD; however, there was no evidence to suggest that these developmental effects were secondary to maternal toxicity.
In pregnant rats injected intravenously with aripiprazole during organogenesis at doses of 3, 9, and 27 mg/kg/day, which are 1, 3, and 9 times the MRHD of 30 mg/day based on mg/m2 body surface area, decreased fetal weight and delayed skeletal ossification were observed at 9 times the MRHD; this dose also caused maternal toxicity.
In pregnant rabbits treated orally with aripiprazole during organogenesis at doses of 10, 30, and 100 mg/kg/day which are 6, 19, and 65 times the MRHD of 30 mg/day based on mg/m2 body surface area, decreased maternal food consumption, and increased abortions as well as increased fetal mortality were observed at 65 times the MRHD. Decreased fetal weight and increased incidence of fused sternebrae were observed at 19 and 65 times the MRHD.
In pregnant rabbits injected intravenously with aripiprazole during organogenesis at doses of 3, 10, and 30 mg/kg/day, which are 2, 6, and 19 times the MRHD of 30 mg/day based on mg/m2 body surface area, decreased fetal weight, increased fetal abnormalities (primarily skeletal), and decreased fetal skeletal ossification were observed at 19 times the MRHD; this dose also caused maternal toxicity. The fetal no-effect dose was 10 mg/kg/day, which is 6 times the MRHD.
In rats treated orally with aripiprazole peri- and postnatally from gestation Day 17 through postpartum Day 21 at doses of 3, 10, and 30 mg/kg/day which are 1, 3, and 10 times the MRHD of 30 mg/day based on mg/m2 body surface area slight maternal toxicity and slightly prolonged gestation were observed at 10 times the MRHD. An increase in stillbirths and, decreases in pup weight (persisting into adulthood) and survival were also seen at this dose.
In rats injected intravenously with aripiprazole from gestation Day 6 through lactation Day 20 at doses of 3, 8, and 20 mg/kg/day, which are 1, 3, and 6 times the MRHD of 30 mg/day based on mg/m2 body surface area, increased stillbirths were observed at 3 and 6 times the MRHD; and decreases in early postnatal pup weight and survival were observed at 6 times the MRHD; these doses also caused some maternal toxicity. There were no effects on postnatal behavioral and reproductive development.
8.2 Lactation
Risk Summary
Limited data from published literature report the presence of aripiprazole in human breast milk, at relative infant doses ranging between 0.7% to 8.3% of the maternal weight-adjusted dosage. There are reports of poor weight gain in breastfed infants exposed to aripiprazole and reports of inadequate milk supply in lactating women taking aripiprazole.
The development and health benefits of breastfeeding should be considered along with the mother's clinical need for ABILIFY and any potential adverse effects on the breastfed infant from ABILIFY or from the underlying maternal condition.
8.4 Pediatric Use
Safety and effectiveness in pediatric patients with major depressive disorder or agitation associated with schizophrenia or bipolar mania have not been established.
The pharmacokinetics of aripiprazole and dehydro-aripiprazole in pediatric patients, 10 to 17 years of age, were similar to those in adults after correcting for the differences in body weight [see Clinical Pharmacology (12.3)].
Schizophrenia
Safety and effectiveness in pediatric patients with schizophrenia were established in a 6 week, placebo-controlled clinical trial in 202 pediatric patients aged 13 to 17 years [see Dosage and Administration (2.1), Adverse Reactions (6.1), and Clinical Studies (14.1)]. Although maintenance efficacy in pediatric patients has not been systematically evaluated, maintenance efficacy can be extrapolated from adult data along with comparisons of aripiprazole pharmacokinetic parameters in adult and pediatric patients.
Bipolar I Disorder
Safety and effectiveness in pediatric patients with bipolar mania were established in a 4 week, placebo-controlled clinical trial in 197 pediatric patients aged 10 to 17 years [see Dosage and Administration (2.2), Adverse Reactions (6.1), and Clinical Studies (14.2)]. Although maintenance efficacy in pediatric patients has not been systematically evaluated, maintenance efficacy can be extrapolated from adult data along with comparisons of aripiprazole pharmacokinetic parameters in adult and pediatric patients.
The efficacy of adjunctive ABILIFY with concomitant lithium or valproate in the treatment of manic or mixed episodes in pediatric patients has not been systematically evaluated. However, such efficacy and lack of pharmacokinetic interaction between aripiprazole and lithium or valproate can be extrapolated from adult data, along with comparisons of aripiprazole pharmacokinetic parameters in adult and pediatric patients.
Irritability Associated with Autistic Disorder
Safety and effectiveness in pediatric patients demonstrating irritability associated with autistic disorder were established in two 8 week, placebo- controlled clinical trials in 212 pediatric patients aged 6 to 17 years [see Indications and Usage (1), Dosage and Administration (2.4), Adverse Reactions (6.1), and Clinical Studies (14.4)]. A maintenance trial was conducted in pediatric patients (6 to 17 years of age) with irritability associated with autistic disorder. The first phase of this trial was an open-label, flexibly dosed (aripiprazole 2 to 15 mg/day) phase in which patients were stabilized (defined as >25% improvement on the ABC-I subscale, and a CGI-I rating of "much improved" or "very much improved") on ABILIFY for 12 consecutive weeks. Overall, 85 patients were stabilized and entered the second, 16 week, double- blind phase where they were randomized to either continue ABILIFY treatment or switch to placebo. In this trial, the efficacy of ABILIFY for the maintenance treatment of irritability associated with autistic disorder was not established.
Tourette's Disorder
Safety and effectiveness of aripiprazole in pediatric patients with Tourette's Disorder were established in one 8 week (aged 7 to 17 years) and one 10 week trial (aged 6 to 18 years) in 194 pediatric patients [see Dosage and Administration (2.5), Adverse Reactions (6.1), and Clinical Studies (14.5)]. Maintenance efficacy in pediatric patients has not been systematically evaluated.
Juvenile Animal Studies
Aripiprazole in juvenile rats caused mortality, CNS clinical signs, impaired memory and learning, and delayed sexual maturation when administered at oral doses of 10, 20, 40 mg/kg/day from weaning (21 days old) through maturity (80 days old). At 40 mg/kg/day, mortality, decreased activity, splayed hind limbs, hunched posture, ataxia, tremors and other CNS signs were observed in both genders. In addition, delayed sexual maturation was observed in males. At all doses and in a dose-dependent manner, impaired memory and learning, increased motor activity, and histopathology changes in the pituitary (atrophy), adrenals (adrenocortical hypertrophy), mammary glands (hyperplasia and increased secretion), and female reproductive organs (vaginal mucification, endometrial atrophy, decrease in ovarian corpora lutea) were observed. The changes in female reproductive organs were considered secondary to the increase in prolactin serum levels. A No Observed Adverse Effect Level (NOAEL) could not be determined and, at the lowest tested dose of 10 mg/kg/day, there is no safety margin relative to the systemic exposures (AUC0-24) for aripiprazole or its major active metabolite in adolescents at the maximum recommended pediatric dose of 15 mg/day. All drug-related effects were reversible after a 2 month recovery period, and most of the drug effects in juvenile rats were also observed in adult rats from previously conducted studies.
Aripiprazole in juvenile dogs (2 months old) caused CNS clinical signs of tremors, hypoactivity, ataxia, recumbency and limited use of hind limbs when administered orally for 6 months at 3, 10, 30 mg/kg/day. Mean body weight and weight gain were decreased up to 18% in females in all drug groups relative to control values. A NOAEL could not be determined and, at the lowest tested dose of 3 mg/kg/day, there is no safety margin relative to the systemic exposures (AUC0-24) for aripiprazole or its major active metabolite in adolescents at the maximum recommended pediatric dose of 15 mg/day. All drug-related effects were reversible after a 2 month recovery period.
8.5 Geriatric Use
No dosage adjustment is recommended for elderly patients [see Boxed Warning, Warnings and Precautions (5.1), and Clinical Pharmacology (12.3)].
Of the 13,543 patients treated with oral ABILIFY in clinical trials, 1,073 (8%) were ≥65 years old and 799 (6%) were ≥75 years old. Placebo-controlled studies of oral ABILIFY in schizophrenia, bipolar mania, or major depressive disorder did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently from younger patients.
Of the 749 patients treated with ABILIFY injection in clinical trials, 99 (13%) were ≥65 years old and 78 (10%) were ≥75 years old. Placebo-controlled studies of ABILIFY injection in patients with agitation associated with schizophrenia or bipolar mania did not include sufficient numbers of patients aged 65 years and over to determine whether they respond differently from younger patients.
ABILIFY is not approved for the treatment of patients with psychosis associated with Alzheimer's disease [see Boxed Warning and Warnings and Precautions (5.1)].
8.6 CYP2D6 Poor Metabolizers
Dosage adjustment is recommended in known CYP2D6 poor metabolizers due to high aripiprazole concentrations. Approximately 8% of Caucasians and 3 to 8% of Black/African Americans cannot metabolize CYP2D6 substrates and are classified as poor metabolizers (PM) [see Dosage and Administration (2.7) and Clinical Pharmacology (12.3)].
8.7 Hepatic and Renal Impairment
No dosage adjustment for ABILIFY is required on the basis of a patient's hepatic function (mild to severe hepatic impairment, Child-Pugh score between 5 and 15), or renal function (mild to severe renal impairment, glomerular filtration rate between 15 and 90 mL/minute) [see Clinical Pharmacology (12.3)].
8.8 Other Specific Populations
No dosage adjustment for ABILIFY is required on the basis of a patient's sex, race, or smoking status [see Clinical Pharmacology (12.3)].
Pregnancy: May cause extrapyramidal and/or withdrawal symptoms in neonates with third trimester exposure (8.1)
DRUG ABUSE AND DEPENDENCE SECTION
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
ABILIFY is not a controlled substance.
9.2 Abuse
ABILIFY has not been systematically studied in humans for its potential for abuse, tolerance, or physical dependence. Consequently, patients should be evaluated carefully for a history of drug abuse, and such patients should be observed closely for signs of ABILIFY misuse or abuse (e.g., development of tolerance, increases in dose, drug-seeking behavior).
9.3 Dependence
In physical dependence studies in monkeys, withdrawal symptoms were observed upon abrupt cessation of dosing. While the clinical trials did not reveal any tendency for any drug-seeking behavior, these observations were not systematic and it is not possible to predict on the basis of this limited experience the extent to which a CNS-active drug will be misused, diverted, and/or abused once marketed.
OVERDOSAGE SECTION
10 OVERDOSAGE
MedDRA terminology has been used to classify the adverse reactions.
10.1 Human Experience
In clinical trials and in postmarketing experience, adverse reactions of deliberate or accidental overdosage with oral ABILIFY have been reported worldwide. These include overdoses with oral ABILIFY alone and in combination with other substances. No fatality was reported with ABILIFY alone. The largest known dose with a known outcome involved acute ingestion of 1,260 mg of oral ABILIFY (42 times the maximum recommended daily dose) by a patient who fully recovered. Deliberate or accidental overdosage was also reported in children (age 12 years and younger) involving oral ABILIFY ingestions up to 195 mg with no fatalities.
Common adverse reactions (reported in at least 5% of all overdose cases) reported with oral ABILIFY overdosage (alone or in combination with other substances) include vomiting, somnolence, and tremor. Other clinically important signs and symptoms observed in one or more patients with ABILIFY overdoses (alone or with other substances) include acidosis, aggression, aspartate aminotransferase increased, atrial fibrillation, bradycardia, coma, confusional state, convulsion, blood creatine phosphokinase increased, depressed level of consciousness, hypertension, hypokalemia, hypotension, lethargy, loss of consciousness, QRS complex prolonged, QT prolonged, pneumonia aspiration, respiratory arrest, status epilepticus, and tachycardia.
10.2 Management of Overdosage
No specific information is available on the treatment of overdose with ABILIFY. An electrocardiogram should be obtained in case of overdosage and if QT interval prolongation is present, cardiac monitoring should be instituted. Otherwise, management of overdose should concentrate on supportive therapy, maintaining an adequate airway, oxygenation and ventilation, and management of symptoms. Close medical supervision and monitoring should continue until the patient recovers.
Charcoal: In the event of an overdose of ABILIFY, an early charcoal administration may be useful in partially preventing the absorption of aripiprazole. Administration of 50 g of activated charcoal, one hour after a single 15 mg oral dose of ABILIFY, decreased the mean AUC and Cmax of aripiprazole by 50%.
Hemodialysis: Although there is no information on the effect of hemodialysis in treating an overdose with ABILIFY, hemodialysis is unlikely to be useful in overdose management since aripiprazole is highly bound to plasma proteins.
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The mechanism of action of aripiprazole in schizophrenia or bipolar mania, is unclear. However, the efficacy of aripiprazole in the listed indications could be mediated through a combination of partial agonist activity at D2 and 5-HT1A receptors and antagonist activity at 5-HT2A receptors.
12.2 Pharmacodynamics
Aripiprazole exhibits high affinity for dopamine D2 and D3, serotonin 5-HT1A and 5-HT2A receptors (Ki values of 0.34 nM, 0.8 nM, 1.7 nM, and 3.4 nM, respectively), moderate affinity for dopamine D4, serotonin 5-HT2C and 5-HT7, alpha1-adrenergic and histamine H1 receptors (Ki values of 44 nM, 15 nM, 39 nM, 57 nM, and 61 nM, respectively), and moderate affinity for the serotonin reuptake site (Ki=98 nM). Aripiprazole has no appreciable affinity for cholinergic muscarinic receptors (IC50>1000 nM).
12.3 Pharmacokinetics
ABILIFY activity is presumably primarily due to the parent drug, aripiprazole, and to a lesser extent, to its major metabolite, dehydro-aripiprazole, which has been shown to have affinities for D2 receptors similar to the parent drug and represents 40% of the parent drug exposure in plasma. The mean elimination half-lives are about 75 hours and 94 hours for aripiprazole and dehydro- aripiprazole, respectively. Steady-state concentrations are attained within 14 days of dosing for both active moieties. Aripiprazole accumulation is predictable from single-dose pharmacokinetics. At steady-state, the pharmacokinetics of aripiprazole is dose-proportional. Elimination of aripiprazole is mainly through hepatic metabolism involving two P450 isozymes, CYP2D6 and CYP3A4. For CYP2D6 poor metabolizers, the mean elimination half- life for aripiprazole is about 146 hours.
Pharmacokinetic studies showed that ABILIFY DISCMELT Orally Disintegrating Tablets are bioequivalent to ABILIFY Tablets.
Oral administration
Absorption
Tablet: Aripiprazole is well absorbed after administration of the tablet, with peak plasma concentrations occurring within 3 hours to 5 hours; the absolute oral bioavailability of the tablet formulation is 87%. ABILIFY can be administered with or without food. Administration of a 15 mg ABILIFY tablet with a standard high-fat meal did not significantly affect the Cmax or AUC of aripiprazole or its active metabolite, dehydro-aripiprazole, but delayed Tmax by 3 hours for aripiprazole and 12 hours for dehydro-aripiprazole.
Oral Solution: Aripiprazole is well absorbed when administered orally as the solution. At equivalent doses, the plasma concentrations of aripiprazole from the solution were higher than that from the tablet formulation. In a relative bioavailability study comparing the pharmacokinetics of 30 mg aripiprazole as the oral solution to 30 mg aripiprazole tablets in healthy subjects, the solution to tablet ratios of geometric mean Cmax and AUC values were 122% and 114%, respectively [see Dosage and Administration (2.6)]. The single-dose pharmacokinetics of aripiprazole were linear and dose-proportional between the doses of 5 mg to 30 mg.
Distribution
The steady-state volume of distribution of aripiprazole following intravenous administration is high (404 L or 4.9 L/kg), indicating extensive extravascular distribution. At therapeutic concentrations, aripiprazole and its major metabolite are greater than 99% bound to serum proteins, primarily to albumin. In healthy human volunteers administered 0.5 to 30 mg/day aripiprazole for 14 days, there was dose-dependent D2 receptor occupancy indicating brain penetration of aripiprazole in humans.
Elimination
Metabolism
Aripiprazole is metabolized primarily by three biotransformation pathways: dehydrogenation, hydroxylation, and N-dealkylation. Based on in vitro studies, CYP3A4 and CYP2D6 enzymes are responsible for dehydrogenation and hydroxylation of aripiprazole, and N-dealkylation is catalyzed by CYP3A4. Aripiprazole is the predominant drug moiety in the systemic circulation. At steady-state, dehydro-aripiprazole, the active metabolite, represents about 40% of aripiprazole AUC in plasma.
Excretion
Following a single oral dose of [14C]-labeled aripiprazole, approximately 25% and 55% of the administered radioactivity was recovered in the urine and feces, respectively. Less than 1% of unchanged aripiprazole was excreted in the urine and approximately 18% of the oral dose was recovered unchanged in the feces.
Drug Interaction Studies
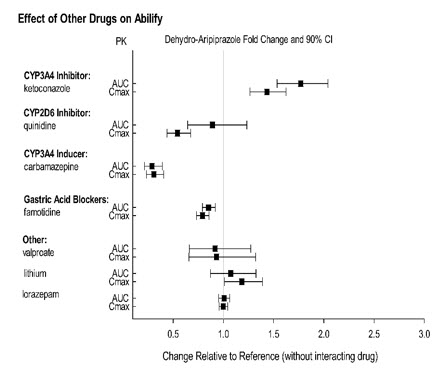
Effect of other drugs on the exposures of aripiprazole and dehydro- aripiprazole are summarized in Figure 1 and Figure 2, respectively. Based on simulation, a 4.5 fold increase in mean Cmax and AUC values at steady-state is expected when extensive metabolizers of CYP2D6 are administered with both strong CYP2D6 and CYP3A4 inhibitors. A 3 fold increase in mean Cmax and AUC values at steady-state is expected in poor metabolizers of CYP2D6 administered with strong CYP3A4 inhibitors.
Figure 1: The Effect of Other Drugs on Aripiprazole Pharmacokinetics
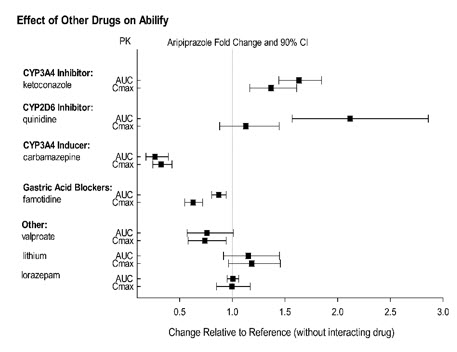
Figure 2: The Effect of Other Drugs on Dehydro-Aripiprazole Pharmacokinetics
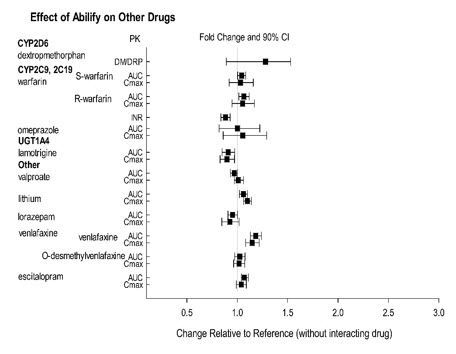
The effect of ABILIFY on the exposures of other drugs are summarized in Figure 3. A population PK analysis in patients with major depressive disorder showed no substantial change in plasma concentrations of fluoxetine (20 or 40 mg/day), paroxetine CR (37.5 or 50 mg/day), or sertraline (100 or 150 mg/day) dosed to steady-state. The steady-state plasma concentrations of fluoxetine and norfluoxetine increased by about 18% and 36%, respectively, and concentrations of paroxetine decreased by about 27%. The steady-state plasma concentrations of sertraline and desmethylsertraline were not substantially changed when these antidepressant therapies were coadministered with aripiprazole.
Figure 3: The Effect of ABILIFY on Pharmacokinetics of Other Drugs
Specific Populations
Exposure of aripiprazole and dehydro-aripiprazole in specific populations are summarized in Figure 4 and Figure 5, respectively. In addition, in pediatric patients (10 to 17 years of age) administered with ABILIFY (20 mg to 30 mg), the body weight corrected aripiprazole clearance was similar to the adults.
Figure 4: Effect of Intrinsic Factors on Aripiprazole Pharmacokinetics
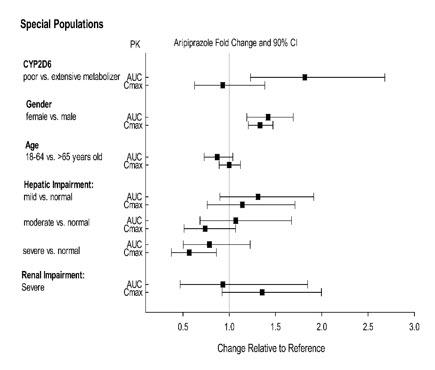
Figure 5: Effect of Intrinsic Factors on Dehydro-Aripiprazole Pharmacokinetics
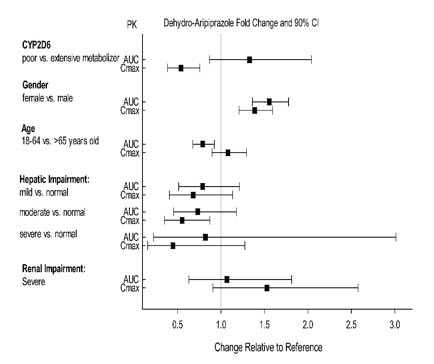
Intramuscular administration
In two pharmacokinetic studies of aripiprazole injection administered intramuscularly to healthy subjects, the median times to the peak plasma concentrations were at 1 hour and 3 hours. A 5 mg intramuscular injection of aripiprazole had an absolute bioavailability of 100%. The geometric mean maximum concentration achieved after an intramuscular dose was on average 19% higher than the Cmax of the oral tablet. While the systemic exposure over 24 hours was generally similar between aripiprazole injection given intramuscularly and after oral tablet administration, the aripiprazole AUC in the first 2 hours after an intramuscular injection was 90% greater than the AUC after the same dose as a tablet. In stable patients with schizophrenia or schizoaffective disorder, the pharmacokinetics of aripiprazole after intramuscular administration were linear over a dose range of 1 mg to 45 mg. Although the metabolism of aripiprazole injection was not systematically evaluated, the intramuscular route of administration would not be expected to alter the metabolic pathways.
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Discuss the following issues with patients prescribed ABILIFY:
Clinical Worsening of Depression and Suicide Risk
Patients, their families, and their caregivers should be encouraged to be alert to the emergence of anxiety, agitation, panic attacks, insomnia, irritability, hostility, aggressiveness, impulsivity, akathisia (psychomotor restlessness), hypomania, mania, other unusual changes in behavior, worsening of depression, and suicidal ideation, especially early during antidepressant treatment and when the dose is adjusted up or down. Families and caregivers of patients should be advised to look for the emergence of such symptoms on a day-to-day basis, since changes may be abrupt. Such symptoms should be reported to the patient's prescriber or health professional, especially if they are severe, abrupt in onset, or were not part of the patient's presenting symptoms. Symptoms such as these may be associated with an increased risk for suicidal thinking and behaviorand indicate a need for very close monitoring and possibly changes in the medication[see Warnings and Precautions (5.3)].
Prescribers or other health professionals should inform patients, their families, and their caregivers about the benefits and risks associated with treatment with ABILIFY and should counsel them in its appropriate use. A patient Medication Guide including information about "Antidepressant Medicines, Depression and other Serious Mental Illness, and Suicidal Thoughts or Actions" is available for ABILIFY. The prescriber or health professional should instruct patients, their families, and their caregivers to read the Medication Guide and should assist them in understanding its contents. Patients should be given the opportunity to discuss the contents of the Medication Guide and to obtain answers to any questions they may have. It should be noted that ABILIFY is not approved as a single agent for treatment of depression and has not been evaluated in pediatric major depressive disorder.
Pathological Gambling and Other Compulsive Behaviors
Advise patients and their caregivers of the possibility that they may experience compulsive urges to shop, intense urges to gamble, compulsive sexual urges, binge eating and/or other compulsive urges and the inability to control these urges while taking aripiprazole. In some cases, but not all, the urges were reported to have stopped when the dose was reduced or stopped [see Warnings and Precautions (5.7)].
Use of Orally Disintegrating Tablet
Do not open the blister until ready to administer. For single tablet removal, open the package and peel back the foil on the blister to expose the tablet. Do not push the tablet through the foil because this could damage the tablet. Immediately upon opening the blister, using dry hands, remove the tablet and place the entire ABILIFY DISCMELT Orally Disintegrating Tablet on the tongue. Tablet disintegration occurs rapidly in saliva. It is recommended that ABILIFY DISCMELT be taken without liquid. However, if needed, it can be taken with liquid. Do not attempt to split the tablet.
Interference with Cognitive and Motor Performance
Because ABILIFY may have the potential to impair judgment, thinking, or motor skills, patients should be cautioned about operating hazardous machinery, including automobiles, until they are reasonably certain that ABILIFY therapy does not affect them adversely [see Warnings and Precautions (5.12)].
Concomitant Medication
Patients should be advised to inform their physicians if they are taking, or plan to take, any prescription or over-the-counter drugs, since there is a potential for interactions [see Drug Interactions (7)].
Heat Exposure and Dehydration
Patients should be advised regarding appropriate care in avoiding overheating and dehydration [see Warnings and Precautions (5.13)].
Sugar Content
Patients should be advised that each mL of ABILIFY Oral Solution contains 400 mg of sucrose and 200 mg of fructose.
Pregnancy
Advise patients to notify their healthcare provider if they become pregnant or intend to become pregnant during treatment with ABILIFY. Advise patients that ABILIFY may cause extrapyramidal and/or withdrawal symptoms (agitation, hypertonia, hypotonia, tremor, somnolence, respiratory distress, and feeding disorder) in a neonate. Advise patients that there is a pregnancy registry that monitors pregnancy outcomes in women exposed to ABILIFY during pregnancy [see Use in Specific Populations (8.1)].
Phenylketonurics
Phenylalanine is a component of aspartame. Each ABILIFY DISCMELT Orally Disintegrating Tablet contains the following amounts: 10 mg, 1.12 mg phenylalanine and 15 mg, 1.68 mg phenylalanine.
DESCRIPTION SECTION
11 DESCRIPTION
Aripiprazole is an atypical antipsychotic drug that is available as ABILIFY® (aripiprazole) Tablets, ABILIFY DISCMELT® (aripiprazole) Orally Disintegrating Tablets, ABILIFY® (aripiprazole) Oral Solution, and ABILIFY® (aripiprazole) Injection, a solution for intramuscular injection. Aripiprazole is 7-[4-[4-(2,3-dichlorophenyl)-1-piperazinyl]butoxy]-3,4-dihydrocarbostyril. The empirical formula is C23H27Cl2N3O2 and its molecular weight is 448.38. The chemical structure is:
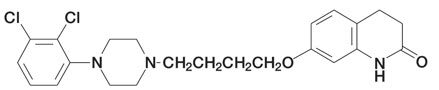
ABILIFY Tablets are available in 2 mg, 5 mg, 10 mg, 15 mg, 20 mg, and 30 mg strengths. Inactive ingredients include cornstarch, hydroxypropyl cellulose, lactose monohydrate, magnesium stearate, and microcrystalline cellulose. Colorants include ferric oxide (yellow or red) and FD&C Blue No. 2 Aluminum Lake.
ABILIFY DISCMELT Orally Disintegrating Tablets are available in 10 mg and 15 mg strengths. Inactive ingredients include acesulfame potassium, aspartame, calcium silicate, croscarmellose sodium, crospovidone, crème de vanilla (natural and artificial flavors), magnesium stearate, microcrystalline cellulose, silicon dioxide, tartaric acid, and xylitol. Colorants include ferric oxide (yellow or red) and FD&C Blue No. 2 Aluminum Lake.
ABILIFY Oral Solution is a clear, colorless to light-yellow solution available in a concentration of 1 mg/mL. The inactive ingredients for this solution include disodium edetate, fructose, glycerin, dl-lactic acid, methylparaben, propylene glycol, propylparaben, sodium hydroxide, sucrose, and purified water. The oral solution is flavored with natural orange cream and other natural flavors.
ABILIFY Injection is available in single-dose vials as a ready-to-use, 9.75 mg/1.3 mL (7.5 mg/mL) clear, colorless, sterile, aqueous solution for intramuscular use only. Inactive ingredients for this solution include 199.5 mg of sulfobutylether β-cyclodextrin (SBECD), 10.4 mg of tartaric acid, q.s. to pH 4.3 of sodium hydroxide, and q.s. to 1.33 mL of water for injection.
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Lifetime carcinogenicity studies were conducted in ICR mice, F344 rats, and Sprague-Dawley (SD) rats. Aripiprazole was administered for 2 years in the diet at doses of 1, 3, 10, and 30 mg/kg/day to ICR mice and 1, 3, and 10 mg/kg/day to F344 rats (0.2, 0.5, 2 and 5 times and 0.3, 1 and 3 times the MRHD of 30 mg/day based on mg/m2 body surface area, respectively). In addition, SD rats were dosed orally for 2 years at 10, 20, 40, and 60 mg/kg/day, which are 3, 6, 13 and 19 times the MRHD based on mg/m2 body surface area. Aripiprazole did not induce tumors in male mice or male rats. In female mice, the incidences of pituitary gland adenomas and mammary gland adenocarcinomas and adenoacanthomas were increased at dietary doses of 3 to 30 mg/kg/day (0.5 to 5 times the MRHD). In female rats, the incidence of mammary gland fibroadenomas was increased at a dietary dose of 10 mg/kg/day (3 times the MRHD); and the incidences of adrenocortical carcinomas and combined adrenocortical adenomas/carcinomas were increased at an oral dose of 60 mg/kg/day (19 times the MRHD).
An increase in mammary, pituitary, and endocrine pancreas neoplasms has been found in rodents after chronic administration of other antipsychotic drugs and is considered to be mediated by prolonged dopamine D2-receptor antagonism and hyperprolactinemia. Serum prolactin was not measured in the aripiprazole carcinogenicity studies. However, increases in serum prolactin levels were observed in female mice in a 13 week dietary study at the doses associated with mammary gland and pituitary tumors. Serum prolactin was not increased in female rats in 4 week and 13 week dietary studies at the dose associated with mammary gland tumors. The relevance for human risk of the findings of prolactin-mediated endocrine tumors in rodents is unclear.
Mutagenesis
The mutagenic potential of aripiprazole was tested in the in vitro bacterial reverse-mutation assay, the in vitro bacterial DNA repair assay, the in vitro forward gene mutation assay in mouse lymphoma cells, the in vitro chromosomal aberration assay in Chinese hamster lung (CHL) cells, the in vivo micronucleus assay in mice, and the unscheduled DNA synthesis assay in rats. Aripiprazole and a metabolite (2,3-DCPP) were clastogenic in the in vitro chromosomal aberration assay in CHL cells with and without metabolic activation. The metabolite, 2,3-DCPP, increased numerical aberrations in the in vitro assay in CHL cells in the absence of metabolic activation. A positive response was obtained in the in vivo micronucleus assay in mice; however, the response was due to a mechanism not considered relevant to humans.
Impairment of Fertility
Female rats were treated orally with aripiprazole from 2 weeks prior to mating through gestation Day 7 at doses of 2, 6, and 20 mg/kg/day, which are 0.6, 2, and 6 times the MRHD of 30 mg/day based on mg/m2 body surface area. Estrus cycle irregularities and increased corpora lutea were seen at all doses, but no impairment of fertility was seen. Increased pre-implantation loss was seen at 2 and 6 times the MRHD, and decreased fetal weight was seen at 6 times the MRHD.
Male rats were treated orally with aripiprazole from 9 weeks prior to mating through mating at doses of 20, 40, and 60 mg/kg/day, which are 6, 13, and 19 times the MRHD of 30 mg/day based on mg/m2 body surface area. Disturbances in spermatogenesis were seen at 19 times the MRHD and prostate atrophy was seen at 13 and 19 times the MRHD without impairment of fertility.
13.2 Animal Toxicology and/or Pharmacology
Aripiprazole produced retinal degeneration in albino rats in a 26 week chronic toxicity study at a dose of 60 mg/kg/day and in a 2 year carcinogenicity study at doses of 40 and 60 mg/kg/day which are 13 and 19 times the MRHD of 30 mg/day based on mg/m2 body surface area. Evaluation of the retinas of albino mice and of monkeys did not reveal evidence of retinal degeneration. Additional studies to further evaluate the mechanism have not been performed. The relevance of this finding to human risk is unknown.
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
ABILIFY® (aripiprazole) Tablets have markings on one side an are available in the strengths and packages listed in Table 32.
Table 32: ABILIFY Tablet Presentations|
Tablet Strength |
Tablet Color/Shape |
Tablet Markings |
Pack Size |
NDC Code |
|---|---|---|---|---|
|
2 mg |
green |
"A-006" and "2" |
Bottle of 30 |
59148-006-13 |
|
5 mg |
blue |
"A-007" and "5" |
Bottle of 30 |
59148-007-13 |
|
10 mg |
pink |
"A-008" and "10" |
Bottle of 30 |
59148-008-13 |
|
15 mg |
yellow |
"A-009" and "15" |
Bottle of 30 |
59148-009-13 |
|
20 mg |
white |
"A-010" and "20" |
Bottle of 30 |
59148-010-13 |
|
30 mg |
pink |
"A-011" and "30" |
Bottle of 30 |
59148-011-13 |
ABILIFY DISCMELT® (aripiprazole) Orally Disintegrating Tablets are round tablets with markings on either side. ABILIFY DISCMELT is available in the strengths and packages listed in Table 33.
Table 33: ABILIFY DISCMELT Orally Disintegrating Tablet Presentations|
Tablet Strength |
Tablet Color |
Tablet Markings |
Pack Size |
NDC Code |
|---|---|---|---|---|
|
10 mg |
pink (with scattered specks) |
"A" and "640" |
Blister of 30 |
59148-640-23 |
|
15 mg |
yellow (with scattered specks) |
"A" and "641" |
Blister of 30 |
59148-641-23 |
ABILIFY® (aripiprazole) Oral Solution (1 mg/mL) is supplied in child-resistant bottles along with a calibrated oral dosing cup. ABILIFY Oral Solution is available as follows:
|
150 mL bottle |
NDC 59148-013-15 |
ABILIFY® (aripiprazole) Injection for intramuscular use is available as a ready-to-use, 9.75 mg/1.3 mL (7.5 mg/mL) solution in clear, Type 1 glass vials as follows:
|
9.75 mg/1.3 mL single-dose vial |
NDC 59148-016-65 |
16.2 Storage
Tablets
Store at 25°C (77°F); excursions permitted between 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature].
Oral Solution
Store at 25°C (77°F); excursions permitted between 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature]. Opened bottles of ABILIFY Oral Solution can be used for up to 6 months after opening, but not beyond the expiration date on the bottle. The bottle and its contents should be discarded after the expiration date.
Injection
Store at 25ºC (77°F); excursions permitted between 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature]. Protect from light by storing in the original container. Retain in carton until time of use.
SPL UNCLASSIFIED SECTION
Tablets manufactured by Otsuka Pharmaceutical Co., Ltd., Tokyo, 101-8535 Japan
Orally Disintegrating Tablets, Oral Solution, and Injection manufactured by Bristol-Myers Squibb Company, Princeton, NJ 08543 USA
Distributed and marketed by Otsuka America Pharmaceutical, Inc., Rockville, MD 20850 USA
ABILIFY is a registered trademark of Otsuka Pharmaceutical Co., Ltd.
© 2022, Otsuka Pharmaceutical Co., Ltd., Tokyo, 101-8535 Japan
SPL MEDGUIDE SECTION
|
MEDICATION GUIDE | |||
|---|---|---|---|
|
ABILIFY® (a BIL ĭ fī) |
ABILIFY® (a BIL ĭ fī) |
ABILIFY® (a BIL ĭ fī) |
ABILIFY® (a BIL ĭ fī) |
|
This Medication Guide has been approved by the U.S. Food and Drug Administration |
Revised: 08/2019 | ||
|
What is the most important information I should know about ABILIFY? *Increased risk of death in elderly patients with dementia-related psychosis: Medicines like ABILIFY can raise the risk of death in elderly people who have lost touch with reality (psychosis) due to confusion and memory loss (dementia). ABILIFY is not approved for the treatment of patients with dementia-related psychosis. *Risk of suicidal thoughts or actions: Antidepressant medicines, depression and other serious mental illnesses, and suicidal thoughts or actions: *Antidepressant medicines may increase suicidal thoughts or actions in some children, teenagers, and young adults within the first few months of treatment. *Depression and other serious mental illnesses are the most important causes of suicidal thoughts and actions. Some people may have a particularly high risk of having suicidal thoughts or actions. These include people who have (or have a family history of) bipolar illness (also called manic-depressive illness) or suicidal thoughts or actions. *How can I watch for and try to prevent suicidal thoughts and actions in myself or a family member? * Pay close attention to any changes, especially sudden changes, in mood, behaviors, thoughts, or feelings. This is very important when an antidepressant medicine is started or when the dose is changed. * Call the healthcare provider right away to report new or sudden changes in mood, behavior, thoughts, or feelings. * Keep all follow-up visits with the healthcare provider as scheduled. Call the healthcare provider between visits as needed, especially if you have concerns about symptoms. Call a healthcare provider right away if you or your family member has any of the following symptoms, especially if they are new, worse, or worry you:
What else do I need to know about antidepressant medicines? *Never stop an antidepressant medicine without first talking to a healthcare provider. Stopping an antidepressant medicine suddenly can cause other symptoms. *Antidepressants are medicines used to treat depression and other illnesses. It is important to discuss all the risks of treating depression and also the risks of not treating it. Patients and their families or other caregivers should discuss all treatment choices with the healthcare provider, not just the use of antidepressants. *Antidepressant medicines have other side effects. Talk to the healthcare provider about the side effects of the medicine prescribed for you or your family member. *Antidepressant medicines can interact with other medicines. Know all of the medicines that you or your family member take. Keep a list of all medicines to show the healthcare provider. Do not start new medicines without first checking with your healthcare provider. *Not all antidepressant medicines prescribed for children are FDA approved for use in children. Talk to your child's healthcare provider for more information. | |||
|
What is ABILIFY? *ABILIFY Oral Tablets, Orally-Disintegrating Tablets, and Oral Solution are prescription medicines used to treat: * schizophrenia * manic or mixed episodes that happen with bipolar I disorder * major depressive disorder (MDD) when ABILIFY is used with antidepressant medicines * irritability associated with autistic disorder * Tourette's disorder *ABILIFY Injection is a prescription medicine used to treat: * agitation associated with schizophrenia or bipolar mania It is not known if ABILIFY is safe or effective in children:
| |||
|
Do not take ABILIFY if youare allergic to aripiprazole or any of the ingredients in ABILIFY. See the end of this Medication Guide for a complete list of ingredients in ABILIFY. | |||
|
Before taking ABILIFY, tell your healthcare provider about all your medical conditions, including if you have or had:
Tell your healthcare provider about all the medicines that you take,
including prescription and over-the-counter medicines, vitamins, and herbal
supplements. | |||
|
How should I take ABILIFY?
| |||
|
What should I avoid while taking ABILIFY?
| |||
|
What are the possible side effects of ABILIFY? *See "What is the most important information I should know about ABILIFY?"
*Stroke in elderly people (cerebrovascular problems) that can lead to death
*Neuroleptic malignant syndrome (NMS). Tell your healthcare provider right away if you have some or all of the following symptoms: high fever, stiff muscles, confusion, sweating, changes in pulse, heart rate, and blood pressure. These may be symptoms of a rare and serious condition that can lead to death. Call your healthcare provider right away if you have any of these symptoms.
*Uncontrolled body movements (tardive dyskinesia). ABILIFY may cause movements that you cannot control in your face, tongue, or other body parts. Tardive dyskinesia may not go away, even if you stop receiving ABILIFY. Tardive dyskinesia may also start after you stop receiving ABILIFY.
*Problems with your metabolism such as:
*High blood sugar (hyperglycemia) and diabetes. Increases in blood sugar can happen in some people who take ABILIFY. Extremely high blood sugar can lead to coma or death. If you have diabetes or risk factors for diabetes (such as being overweight or a family history of diabetes), your healthcare provider should check your blood sugar before you start ABILIFY and during your treatment. *Unusual urges. Some people taking ABILIFY have had unusual urges, such as gambling, binge eating or eating that you cannot control (compulsive), compulsive shopping and sexual urges. *Orthostatic hypotension (decreased blood pressure). Lightheadedness or fainting may happen when rising too quickly from a sitting or lying position. *Falls. ABILIFY may make you sleepy or dizzy, may cause a decrease in your blood pressure when changing position and can slow your thinking and motor skills which may lead to falls that can cause fractures or other injuries. *Low white blood cell count *Seizures (convulsions) *Problems with control of your body temperature especially when you exercise a lot or are in an area that is very hot. It is important for you to drink water to avoid dehydration. See "What should I avoid while receiving ABILIFY?" *Difficulty swallowing that can cause food or liquid to get into your lungs. The most common side effects of ABILIFY in adults include: | |||
|
| ||
|
The most common side effects of ABILIFY in children include: | |||
|
| ||
|
These are not all the possible side effects of ABILIFY. | |||
|
How should I store ABILIFY?
Keep ABILIFY and all medicines out of the reach of children. | |||
|
General information about the safe and effective use of ABILIFY | |||
|
What are the ingredients in ABILIFY? |
