Clarinex
These highlights do not include all the information needed to use CLARINEX safely and effectively. See full prescribing information for CLARINEX. CLARINEX (desloratadine) Tablets, RediTabs, and Oral Solution for oral useInitial U.S. Approval: 2001
c671342e-69a2-4ca5-abc2-8166ed4240d4
HUMAN PRESCRIPTION DRUG LABEL
Nov 14, 2022
Organon LLC
DUNS: 117494753
Products 2
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Desloratadine
PRODUCT DETAILS
INGREDIENTS (13)
Desloratadine
PRODUCT DETAILS
INGREDIENTS (13)
Drug Labeling Information
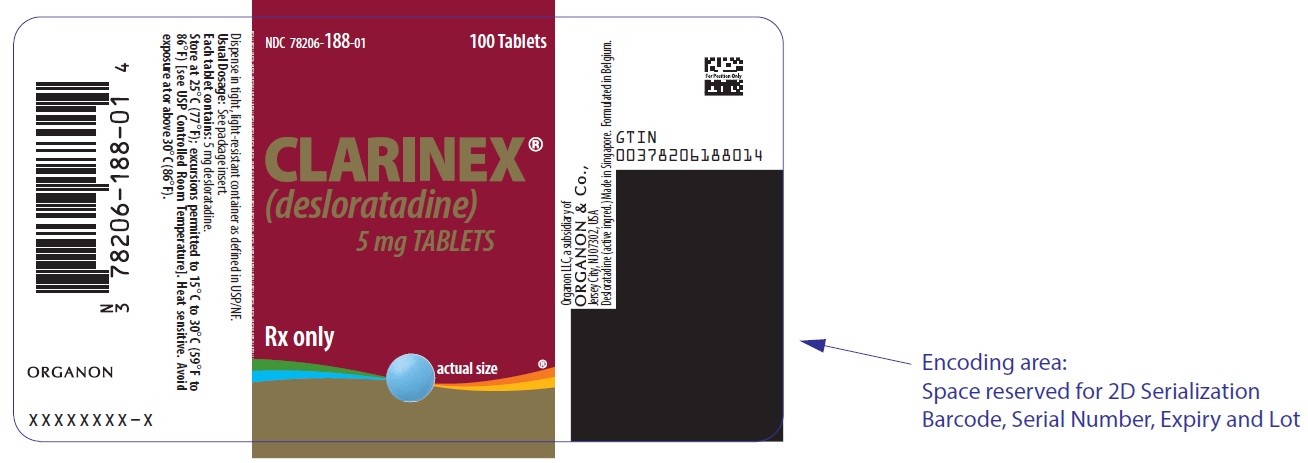
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 100 Tablet Bottle Label
NDC 78206-188-01
100 Tablets
CLARINEX®
(desloratadine)
5 mg TABLETS
Rx only
actual size ®
DESCRIPTION SECTION
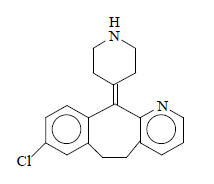
11 DESCRIPTION
CLARINEX (desloratadine) Tablets are light blue, round, film-coated tablets containing 5 mg desloratadine, an antihistamine, to be administered orally. CLARINEX Tablets also contain the following excipients: dibasic calcium phosphate dihydrate USP, microcrystalline cellulose NF, corn starch NF, talc USP, carnauba wax NF, white wax NF, coating material consisting of lactose monohydrate, hypromellose, titanium dioxide, polyethylene glycol, and FD&C Blue #2 Aluminum Lake.
Desloratadine is a white to off-white powder that is slightly soluble in water, but very soluble in ethanol and propylene glycol. It has an empirical formula: C19H19ClN2 and a molecular weight of 310.8. The chemical name is 8-chloro-6,11-dihydro-11-(4-piperdinylidene)-5H-benzo[5,6]cyclohepta[1,2-b]pyridine and has the following structure:
|
|