Cough Out
Cough Out
fe4c3921-8d30-4cd6-ba90-9211b362a38a
HUMAN OTC DRUG LABEL
Jun 18, 2025
Efficient Laboratories Inc.
DUNS: 969044932
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Guaifenesin
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (10)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
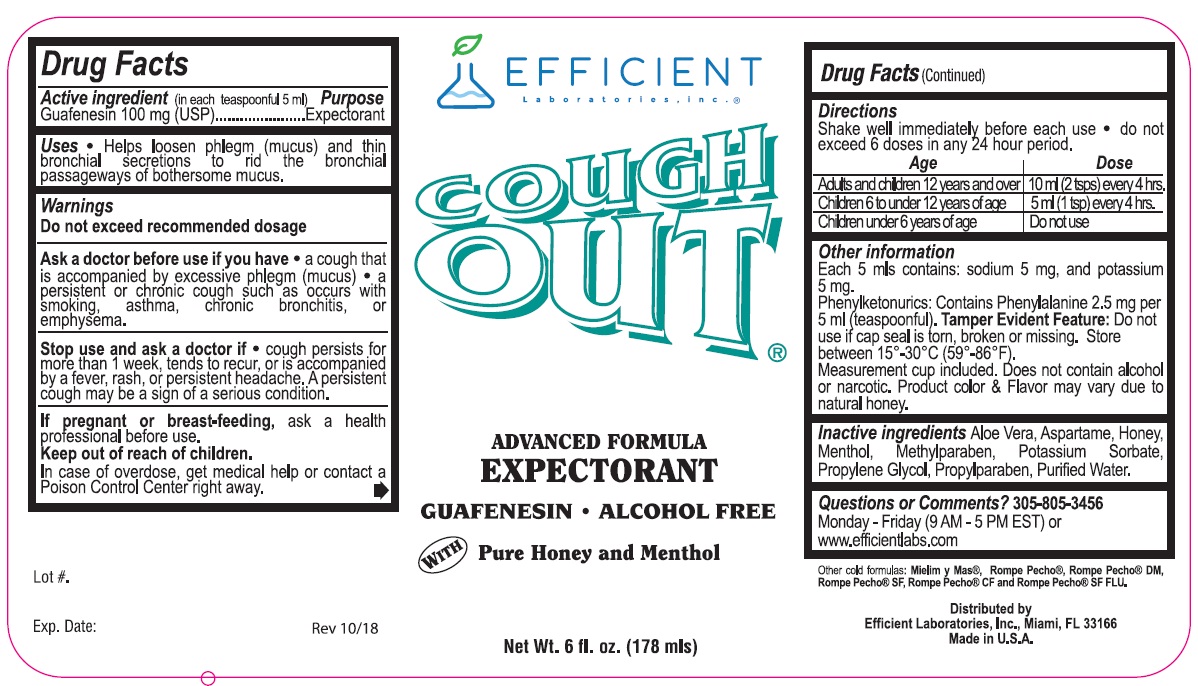
INDICATIONS & USAGE SECTION
**Uses**
Helps loosen phlegm (mucus) and thin bronchial secretions to rid the bronchial passageways of bothersome mucus.
OTC - ACTIVE INGREDIENT SECTION
Active Ingredient: (In each teaspoonful 5 mls) Purpose
Guiafenesin 100 mg (USP)............................................Expectorant
OTC - PURPOSE SECTION
PurposeExpectorant
WARNINGS SECTION
Warnings
**Do not exceed recommended dosage**
**Ask a doctor before use if you have**
- a cough that is accompanied by excessive phlegm (mucus)
- a persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema.
**Stop use and ask a doctor if**
- cough persists for more than 1 week, tends to recur, or is accompanied by a fever, rash, or persstent headached. A persistent cough may be a sign of a serious condition.
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
Keep Out of Reach of Children.
In case of accidental overdose, get medical help or contact the Poison Control Center right away.
OTC - PREGNANCY OR BREAST FEEDING SECTION
**If pregnant or breast-feeding,**ask a health professional before use.
DOSAGE & ADMINISTRATION SECTION
Directions:
Shake well immediately before use.
Do not exceed 6 doses in any 24 hour period.
Adults and Children 12 years and over - 10 ml (2 tsps) every 4 hours
Children 6 to under 12 years of age - 5 ml (1 tsp) every 4 hours
Children under 6 years of age - Do not use
INACTIVE INGREDIENT SECTION
**Inactive Ingredients:**Aloe Vera, Aspartame, Honey, Menthol, Methylparaben, Potassium Sorbate, Propylene Glycol, Propylparaben, Purified Water
OTHER SAFETY INFORMATION
Each 5 mls contains: sodium 5 mg, and potassium 5 mg
Phenylketonurics: Contains Phenylalanine 2.5 mg per 5 ml (teaspoonful).
OTC - QUESTIONS SECTION
Questions or Comments?
305.805.3456 Monday-Friday 9AM to 5PM EST
www.efficientlabs.com
