Mozobil
These highlights do not include all the information needed to use MOZOBIL safely and effectively. See full prescribing information for MOZOBIL. MOZOBIL (plerixafor) injection, for subcutaneous use Initial U.S. Approval: 2008
0ed08d2b-5051-46b2-aa37-1d6275bf9003
HUMAN PRESCRIPTION DRUG LABEL
Sep 22, 2025
Sanofi-Aventis U.S. LLC
DUNS: 824676584
sanofi-aventis U.S. LLC
DUNS: 824676584
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
PLERIXAFOR
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (5)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 24 mg/1.2 mL Vial Carton
NDC 0024-5862-01
Mozobil®
(plerixafor) injection
24 mg/1.2 mL
(20 mg/mL)
For subcutaneous
injection only
One single-dose vial.
Discard unused portion.
Rx only
SANOFI GENZYME
DESCRIPTION SECTION
11 DESCRIPTION
Mozobil (plerixafor) injection is a sterile, preservative-free, clear, colorless to pale-yellow, isotonic solution for subcutaneous injection. Each mL of the sterile solution contains 20 mg of plerixafor. Each single-dose vial is filled to deliver 1.2 mL of the sterile solution that contains 24 mg of plerixafor and 5.9 mg of sodium chloride in Water for Injection adjusted to a pH of 6.0 to 7.5 with hydrochloric acid and with sodium hydroxide, if required.
Plerixafor is a hematopoietic stem cell mobilizer with a chemical name 1,4-Bis((1,4,8,11-tetraazacyclotetradecan-1-yl)methyl)benzene. It has the molecular formula C28H54N8. The molecular weight of plerixafor is 502.79 g/mol. The structural formula is provided in Figure 1.
Figure 1: Structural Formula
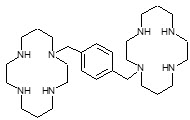
Plerixafor is a white to off-white crystalline solid. It is hygroscopic. Plerixafor has a typical melting point of 131.5°C. The partition coefficient of plerixafor between 1-octanol and pH 7 aqueous buffer is <0.1.
