Acclean Chlorhexidine Gluconate 0.12% Oral Rinse
Henry Schein Acclean 4-oz Chlorhexidine Gluconate 0.12% Oral Rinse, USP
4a2a6bb8-85b0-6c36-e054-00144ff8d46c
HUMAN PRESCRIPTION DRUG LABEL
Sep 6, 2022
Xttrium Laboratories, Inc.
DUNS: 007470579
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Chlorhexidine Gluconate 0.12% Oral Rinse
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC 0404-6720-04
REF 112-6720
ACCLEAN ®
Chlorhexidine Gluconate
0.12% Oral Rinse, USP
Anti-Microbial Rinse,
Mint
DIRECTIONS FOR USE: Swish 1 tablespoon (15 ml) in mouth undiluted for 30 seconds, then**spit out.**Use after breakfast and before bedtime. Or, use as prescribed.
NOTE: To minimize medicinal taste, do not rinse with water immediately after use.
KEEP OUT OF REACH OF CHILDREN
1999HS04EZCD
by HENRY SCHEIN®
4 oz. (118 ml)
INGREDIENTS: 0.12% chlorhexidine gluconate in a base containing water, 11.6% alcohol, glycerin, PEG-40 sorbitan diisostearate, flavor, sodium saccharin, and FD&C Blue No.1.
To open, press down while turning cap. To reseal, turn cap past "clicks" until tightly locked.
CAUTION, CONSULT ACCOMPANYING DOCUMENTS
Rx Only

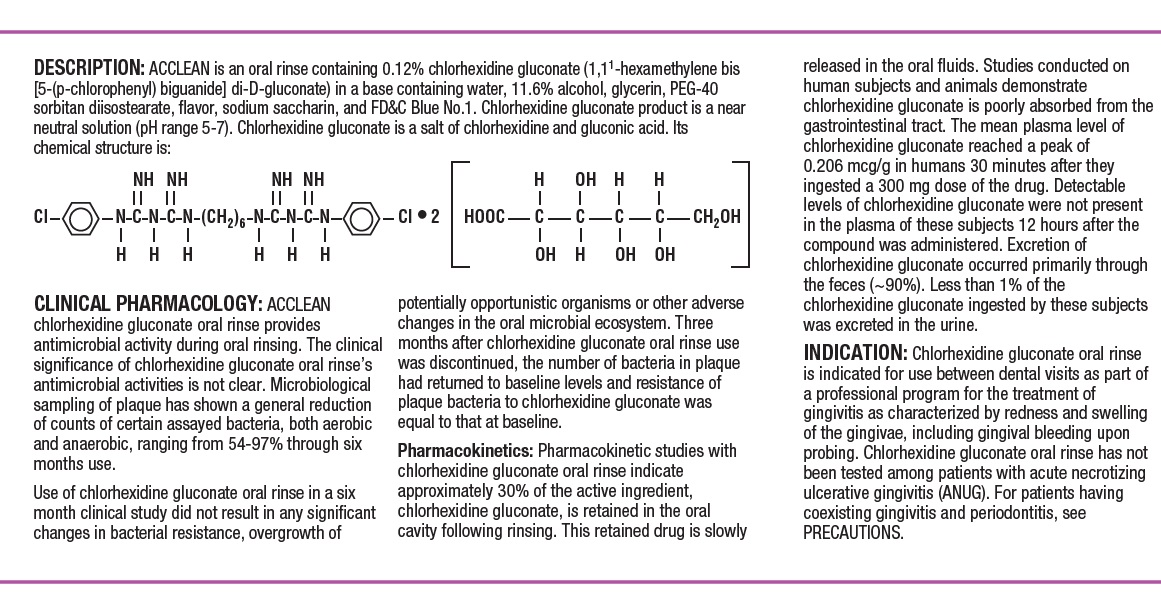


DESCRIPTION SECTION
ACCLEAN is an oral rinse containing 0.12% chlorhexidine gluconate (1,1’-hexamethylene bis [5-(p-chlorphenyl) biguanide]di-D-gluconate) in a base containing water, 11.6% alcohol, glycerin, PEG-40 sorbitan diisostearate, flavor, sodium saccharin and FD&C Blue No. 1. Chlorhexidine gluconate product is a near neutral solution (pH range 5-7). Chlorhexidine gluconate is a salt of chlorhexidine and gluconic acid. Its chemical structure is: