BETIMOL
BETIMOL (timolol ophthalmic solution) 0.25%, 0.5%
3ae775f4-340c-4bf4-aa49-8a31daba0f8b
HUMAN PRESCRIPTION DRUG LABEL
Sep 5, 2023
Thea Pharma Inc.
DUNS: 117787029
Products 2
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
timolol
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (5)
timolol
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (5)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 5 mL Bottle Carton - NDC 82854-002-05
NDC 82584-002-05
BETIMOL ®
(timolol ophthalmic
solution) 0.5%
Timolol equivalent (timolol
hemihydrate 5.12 mg/mL)
Rx Only
5 mL
DESCRIPTION SECTION
DESCRIPTION
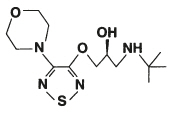
Betimol ® (timolol ophthalmic solution), 0.25% and 0.5%, is a non-selective beta-adrenergic antagonist for ophthalmic use. The chemical name of the active ingredient is (S)-1-[(1, 1-dimethylethyl)amino]-3-[[4-(4-morpholinyl)-1,2,5-thiadiazol-3-yl]oxy]-2-propanol. Timolol hemihydrate is the levo isomer. Specific rotation is [α] 25 405nm=-16° (C=10% as the hemihydrate form in 1N HCl).
|
The molecular formula of timolol is Formula C 13H 24N 4O 3S and its structural formula is: |
|
Timolol (as the hemihydrate) is a white, odorless, crystalline powder which is slightly soluble in water and freely soluble in ethanol. Timolol hemihydrate is stable at room temperature.
Betimol ® is a clear, colorless, isotonic, sterile, microbiologically preserved phosphate buffered aqueous solution.
It is supplied in two dosage strengths, 0.25% and 0.5%.
Each mL of Betimol ® 0.25% contains 2.56 mg of timolol hemihydrate equivalent to 2.5 mg timolol.
Each mL of Betimol ® 0.5% contains 5.12 mg of timolol hemihydrate equivalent to 5.0 mg timolol.
Inactive ingredients: monosodium and disodium phosphate dihydrate to adjust pH (6.5 - 7.5) and water for injection, benzalkonium chloride 0.01 % added as preservative.
The osmolality of Betimol ® is 260 to 320 mOsmol/kg.
WARNINGS SECTION
WARNINGS
As with other topically applied ophthalmic drugs, Betimol ® is absorbed systemically. The same adverse reactions found with systemic administration of beta-adrenergic blocking agents may occur with topical administration. For example, severe respiratory and cardiac reactions, including death due to bronchospasm in patients with asthma, and rarely, death in association with cardiac failure have been reported following systemic or topical administration of beta-adrenergic blocking agents.
Cardiac Failure
Sympathetic stimulation may be essential for support of the circulation in individuals with diminished myocardial contractility, and its inhibition by beta-adrenergic receptor blockade may precipitate more severe cardiac failure.
In patients without a history of cardiac failure, continued depression of the myocardium with beta-blocking agents over a period of time can, in some cases, lead to cardiac failure. Betimol ® should be discontinued at the first sign or symptom of cardiac failure.
Obstructive Pulmonary Disease
Patients with chronic obstructive pulmonary disease (e.g. chronic bronchitis, emphysema) of mild or moderate severity, bronchospastic disease, or a history of bronchospastic disease (other than bronchial asthma or a history of bronchial asthma which are contraindications) should in general not receive beta-blocking agents.
Major Surgery
The necessity or desirability of withdrawal of beta-adrenergic blocking agents prior to a major surgery is controversial. Beta-adrenergic receptor blockade impairs the ability of the heart to respond to beta-adrenergically mediated reflex stimuli. This may augment the risk of general anesthesia in surgical procedures. Some patients receiving beta-adrenergic receptor blocking agents have been subject to protracted severe hypotension during anesthesia. Difficulty in restarting and maintaining the heartbeat has also been reported. For these reasons, in patients undergoing elective surgery, gradual withdrawal of beta-adrenergic receptor blocking agents is recommended. If necessary during surgery, the effects of beta-adrenergic blocking agents may be reversed by sufficient doses of beta-adrenergic agonists.
Diabetes Mellitus
Beta-adrenergic blocking agents should be administered with caution in patients subject to spontaneous hypoglycemia or to diabetic patients (especially those with labile diabetes) who are receiving insulin or oral hypoglycemic agents. Beta-adrenergic receptor blocking agents may mask the signs and symptoms of acute hypoglycemia.
Thyrotoxicosis
Beta-adrenergic blocking agents may mask certain clinical signs (e.g. tachycardia) of hyperthyroidism. Patients suspected of developing thyrotoxicosis should be managed carefully to avoid abrupt withdrawal of beta- adrenergic blocking agents which might precipitate a thyroid storm.
HOW SUPPLIED SECTION
HOW SUPPLIED
Betimol ® (timolol ophthalmic solution) is a clear, colorless solution.
Betimol ® 0.25% is supplied in a white, opaque, plastic, ophthalmic dispenser bottle with a controlled drop tip as follows:
|
NDC 82584-001-05 |
5 mL |
fill in 5 cc container |
Betimol ® 0.5% is supplied in a white, opaque, plastic, ophthalmic dispenser bottle with a controlled drop tip as follows:
|
NDC 82584-002-05 |
5 mL |
fill in 5 cc container |
|
NDC 82584-002-15 |
15 mL |
fill in 15 cc container |
Rx Only
STORAGE
Store between 15° to 25°C (59° to 77°F). Do not freeze. Protect from light.