Rasagiline
These highlights do not include all the information needed to use RASAGILINE TABLETS safely and effectively. See full prescribing information for RASAGILINE TABLETS. RASAGILINE tablets for oral use Initial U.S. Approval: 2006
5dfeec8f-f4f3-4e06-915e-3244467516a6
HUMAN PRESCRIPTION DRUG LABEL
Sep 30, 2022
Ascend Laboratories, LLC
DUNS: 141250469
Products 2
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Rasagiline
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
Rasagiline
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL 1 MG TABLET BOTTLE LABEL
Ascend Laboratories, LLC
NDC 67877-260-30
Bottle of 30 tablets
Rx Only

Indications & Usage Section
1 INDICATIONS AND USAGE
Rasagiline tablets are indicated for the treatment of Parkinson's disease (PD)
Rasagiline tablets, a monoamine oxidase (MAO)-B inhibitor (MAOI), is indicated for the treatment of Parkinson’s disease (1)
Contraindications Section
4 CONTRAINDICATIONS
Rasagiline is contraindicated for use with meperidine, tramadol, methadone,
propoxyphene and MAO inhibitors (MAOIs), including other selective MAO-B
inhibitors, because of risk of serotonin syndrome [See Warnings and Precautions (5.2)]. At least 14 days should elapse between discontinuation of
rasagiline and initiation of treatment with these medications.
Rasagiline is contraindicated for use with St. John’s wort and with
cyclobenzaprine.
Rasagiline is contraindicated for use with dextromethorphan because of risk of
episode of psychosis or bizarre behavior.
Concomitant use of meperidine, tramadol, methadone, propoxyphene dextromethorphan, St. John’s wort, cyclobenzaprine, or another (selective or non-selective) MAO inhibitor (4)
Warnings And Precautions Section
5 WARNINGS AND PRECAUTIONS
5.1 Hypertension
Exacerbation of hypertension may occur during treatment with rasagiline.
Medication adjustment may be necessary if elevation of blood pressure is
sustained. Monitor patients for new onset hypertension or hypertension that is
not adequately controlled after starting rasagiline.
In Study 3, rasagiline (1 mg/day) given in conjunction with levodopa, produced
an increased incidence of significant blood pressure elevation (systolic > 180
or diastolic > 100 mm Hg) of 4% compared to 3% for placebo [see Adverse Reactions (6.1)].
When used as an adjunct to levodopa (Studies 3 and 4), the risk for developing
post-treatment high blood pressure (e.g., systolic > 180 or diastolic >100 mm
Hg) combined with a significant increase from baseline (e.g., systolic > 30 or
diastolic > 20 mm Hg) was higher for rasagiline (2%) compared to placebo (1%).
Dietary tyramine restriction is not required during treatment with recommended
doses of rasagiline. However, certain foods that may contain very high amounts
(i.e., more than 150 mg) of tyramine that could potentially cause severe
hypertension because of tyramine interaction (including various clinical
syndromes referred to as hypertensive urgency, crisis, or emergency) in
patients taking rasagiline, even at the recommended doses, due to increased
sensitivity to tyramine. Patients should be advised to avoid foods containing
a very large amount of tyramine while taking recommended doses of rasagiline
because of the potential for large increases in blood pressure including
clinical syndromes referred to as hypertensive urgency, crisis, or emergency.
Rasagiline is a selective inhibitor of MAO-B at the recommended doses of 0.5
or 1 mg daily. Selectivity for inhibiting MAO-B diminishes in a dose-related
manner as the dose is progressively increased above the recommended daily
doses.
5.2 Serotonin Syndrome
Serotonin syndrome has been reported with concomitant use of an antidepressant
(e.g., selective serotonin reuptake inhibitors-SSRIs, serotonin-norepinephrine
reuptake inhibitors-SNRIs, tricyclic antidepressants, tetracyclic
antidepressants, triazolopyridine antidepressants) and a nonselective MAOI
(e.g., phenelzine, tranylcypromine) or selective MAO-B inhibitors, such as
selegiline (Eldepryl) and rasagiline (Rasagiline tablets). Serotonin syndrome
has also been reported with concomitant use of rasagiline with meperidine,
tramadol, methadone, or propoxyphene. Rasagiline is contraindicated for use
with meperidine, tramadol, methadone, propoxyphene and MAO inhibitors (MAOIs),
including other selective MAO-B inhibitors [see Contraindications (4) and Drug Interactions (7.1, 7.2, 7.3)].
In the postmarketing period, potentially life-threatening serotonin syndrome
has been reported in patients treated with antidepressants concomitantly with
rasagiline. Concomitant use of rasagiline with one of many classes of
antidepressants (e.g., SSRIs, SNRIs, triazolopyridine, tricyclic or
tetracyclic antidepressants) is not recommended [see Drug Interactions (7.5)].
The symptoms of serotonin syndrome have included behavioral and
cognitive/mental status changes (e.g., confusion, hypomania, hallucinations,
agitation, delirium, headache, and coma), autonomic effects (e.g., syncope,
shivering, sweating, high fever/hyperthermia, hypertension, tachycardia,
nausea, diarrhea), and somatic effects (e.g., muscular rigidity, myoclonus,
muscle twitching, hyperreflexia manifested by clonus, and tremor). Serotonin
syndrome can result in death.
Rasagiline clinical trials did not allow concomitant use of fluoxetine or
fluvoxamine with rasagiline, and the potential drug interaction between
rasagiline and antidepressants has not been studied systematically. Although a
small number of rasagiline -treated patients were concomitantly exposed to
antidepressants (tricyclics n=115; SSRIs n=141), the exposure, both in dose
and number of subjects, was not adequate to rule out the possibility of an
untoward reaction from combining these agents. At least 14 days should elapse
between discontinuation of rasagiline and initiation of treatment with a SSRI,
SNRI, tricyclic, tetracyclic, or triazolopyridine antidepressant. Because of
the long half-lives of certain antidepressants (e.g., fluoxetine and its
active metabolite), at least five weeks (perhaps longer, especially if
fluoxetine has been prescribed chronically and/or at higher doses) should
elapse between discontinuation of fluoxetine and initiation of rasagiline [see Drug Interactions (7.5)].
5.3 Falling Asleep During Activities of Daily Living and Somnolence
It has been reported that falling asleep while engaged in activities of daily
living always occurs in a setting of preexisting somnolence, although patients
may not give such a history. For this reason, prescribers should monitor
patients for drowsiness or sleepiness, because some of the events occur well
after initiation of treatment with dopaminergic medication. Prescribers should
also be aware that patients may not acknowledge drowsiness or sleepiness until
directly questioned about drowsiness or sleepiness during specific activities.
Cases of patients treated with rasagiline and other dopaminergic medications
have reported falling asleep while engaged in activities of daily living
including the operation of motor vehicles, which sometimes resulted in
accidents. Although many of these patients reported somnolence while on
rasagiline with other dopaminergic medications, some perceived that they had
no warning signs, such as excessive drowsiness, and believed that they were
alert immediately prior to the event. Some of these events have been reported
more than 1-year after initiation of treatment.
In Study 3, somnolence was a common occurrence in patients receiving
rasagiline and was more frequent in patients with Parkinson’s disease
receiving rasagiline than in respective patients receiving placebo (6%
rasagiline compared to 4% Placebo) [see Adverse Reactions (6.1)]. Before
initiating treatment with rasagiline, patients should be advised of the
potential to develop drowsiness and specifically asked about factors that may
increase the risk with rasagiline such as concomitant sedating medications,
the presence of sleep disorders, and concomitant medications that increase
rasagiline plasma levels (e.g., ciprofloxacin) [see Drug Interactions (7.6)].
If a patient develops significant daytime sleepiness or episodes of falling
asleep during activities that require active participation (e.g., driving a
motor vehicle, conversations, eating), rasagiline should ordinarily be
discontinued. If a decision is made to continue these patients on rasagiline,
advise them to avoid driving and other potentially dangerous activities. There
is insufficient information to establish that dose reduction will eliminate
episodes of falling asleep while engaged in activities of daily living.
5.4 Ciprofloxacin or Other CYP1A2 Inhibitors
Rasagiline plasma concentrations may increase up to 2 fold in patients using concomitant ciprofloxacin and other CYP1A2 inhibitors. Patients taking concomitant ciprofloxacin or other CYP1A2 inhibitors should not exceed a dose of rasagiline 0.5 mg once daily [see Dosage and Administration (2.2), Drug Interactions (7.6), and Clinical Pharmacology (12.3)].
5.5 Hepatic Impairment
Rasagiline plasma concentration may increase in patients with hepatic impairment. Patients with mild hepatic impairment should be given the dose of rasagiline 0.5 mg once daily. Rasagiline should not be used in patients with moderate or severe hepatic impairment [see Dosage and Administration (2.3) and Clinical Pharmacology (12.3)].
5.6 Hypotension / Orthostatic Hypotension
In Study 3, the incidence of orthostatic hypotension consisting of a systolic
blood pressure decrease (≥ 30 mm Hg) or a diastolic blood pressure decrease (≥
20 mm Hg) after standing was 13% with rasagiline (1 mg/day) compared to 9%
with placebo [see Adverse Reactions (6.1)].
At the 1 mg dose, the frequency of orthostatic hypotension (at any time during
the study) was approximately 44% for rasagiline vs 33% for placebo for mild to
moderate systolic blood pressure decrements (≥20 mm Hg), 40% for rasagiline vs
33% for placebo for mild to moderate diastolic blood pressure decrements (≥10
mm Hg), 7% for rasagiline vs 3% for placebo for severe systolic blood pressure
decrements (≥ 40 mm Hg), and 9% for rasagiline vs 6% for placebo for severe
diastolic blood pressure decrements (≥ 20 mm Hg). There was also an increased
risk for some of these abnormalities at the lower 0.5 mg daily dose and for an
individual patient having mild to moderate or severe orthostatic hypotension
for both systolic and diastolic blood pressure.
In Study 2 where rasagiline was given as an adjunct therapy in patients not
taking concomitant levodopa, there were 5 reports of orthostatic hypotension
in patients taking rasagiline 1 mg (3.1%) and 1 report in patients taking
placebo (0.6%) [see Adverse Reactions(6.1)].
Clinical trial data further suggest that orthostatic hypotension occurs most
frequently in the first two months of rasagiline treatment and tends to
decrease over time.
Some patients treated with rasagiline experienced a mildly increased risk for
significant decreases in blood pressure unrelated to standing but while
supine. The risk for post-treatment hypotension (e.g., systolic < 90 or
diastolic < 50 mm Hg) combined with a significant decrease from baseline
(e.g., systolic > 30 or diastolic > 20 mm Hg) was higher for rasagiline 1 mg
(3.2%) compared to placebo (1.3%).
There was no clear increased risk for lowering of blood pressure or postural
hypotension associated with rasagiline 1 mg/day as monotherapy.
When used as an adjunct to levodopa, postural hypotension was also reported as
an adverse reaction in approximately 6% of patients treated with rasagiline
0.5 mg, 9% of patients treated with rasagiline 1 mg and 3% of patients treated
with placebo. Postural hypotension led to drug discontinuation and premature
withdrawal from clinical trials in one (0.7%) patient treated with rasagiline
1 mg/day, no patients treated with rasagiline 0.5 mg/day and no placebo-
treated patients.
5.7 Dyskinesia
When used as an adjunct to levodopa, rasagiline may cause dyskinesia or potentiate dopaminergic side effects and exacerbate preexisting dyskinesia. In Study 3, the incidence of dyskinesia was 18% for patients treated with 0.5 mg or 1 mg rasagiline as an adjunct to levodopa and 10% for patients treated with placebo as an adjunct to levodopa. Decreasing the dose of levodopa may mitigate this side effect [see Adverse Reactions (6.1)].
5.8 Hallucinations / Psychotic-Like Behavior
In the monotherapy study (Study 1), the incidence of hallucinations reported
as an adverse event was 1.3% in patients treated with rasagiline 1 mg and 0.7%
in patients treated with placebo. In Study 1, the incidence of hallucinations
reported as an adverse reaction and leading to drug discontinuation and
premature withdrawal was 1.3% in patients treated with rasagiline 1 mg and 0%
in placebo-treated patients.
When studied as an adjunct therapy without levodopa (Study 2), hallucinations
were reported as an adverse reaction in 1.2% of patients treated with 1 mg/day
rasagiline and 1.8% of patients treated with placebo. Hallucinations led to
drug discontinuation and premature withdrawal from the clinical trial in 0.6%
of patients treated with rasagiline 1 mg/day and in none of the placebo-
treated patients.
When studied as an adjunct to levodopa (Study 3), the incidence of hallucinations was approximately 5% in patients treated with rasagiline 0.5 mg/day, 4% in patients treated with rasagiline 1 mg/day, and 3% in patients treated with placebo. The incidence of hallucinations leading to drug discontinuation and premature withdrawal was about 1% in patients treated with 0.5 mg rasagiline and 1 mg rasagiline/day, and 0% in placebo-treated patients [see Adverse Reactions (6.1)].
Postmarketing reports indicate that patients may experience new or worsening
mental status and behavioral changes, which may be severe, including
psychotic-like behavior during treatment with rasagiline or after starting or
increasing the dose of rasagiline. Other drugs prescribed to improve the
symptoms of Parkinson’s disease can have similar effects on thinking and
behavior. This abnormal thinking and behavior can consist of one or more of a
variety of manifestations including paranoid ideation, delusions,
hallucinations, confusion, psychotic-like behavior, disorientation, aggressive
behavior, agitation, and delirium. Patients should be informed of the
possibility of developing hallucinations and instructed to report them to
their healthcare provider promptly should they develop.
Patients with a major psychotic disorder should ordinarily not be treated with
rasagiline because of the risk of exacerbating the psychosis with an increase
in central dopaminergic tone. In addition, many treatments for psychosis that
decrease central dopaminergic tone may decrease the effectiveness of
rasagiline [see Drug Interactions (7.8)].
Consider dose reduction or stopping the medication if a patient develops
hallucinations or psychotic like behaviors while taking rasagiline.
5.9 Impulse Control / Compulsive Behaviors
Case reports suggest that patients can experience intense urges to gamble, increased sexual urges, intense urges to spend money, binge eating, and/or other intense urges, and the inability to control these urges while taking one or more of the medications, including rasagiline, that increase central dopaminergic tone and that are generally used for the treatment of Parkinson’s disease. In some cases, although not all, these urges were reported to have stopped when the dose was reduced or the medication was discontinued. Because patients may not recognize these behaviors as abnormal, it is important for prescribers to specifically ask patients or their caregivers about the development of new or increased gambling urges, sexual urges, uncontrolled spending or other urges while being treated with rasagiline. Consider dose reduction or stopping the medication if a patient develops such urges while taking rasagiline.
5.10 Withdrawal-Emergent Hyperpyrexia and Confusion
A symptom complex resembling neuroleptic malignant syndrome (characterized by elevated temperature, muscular rigidity, altered consciousness, and autonomic instability), with no other obvious etiology, has been reported in association with rapid dose reduction, withdrawal of, or changes in drugs that increase central dopaminergic tone.
- May cause hypertension (including severe hypertensive syndromes) at recommended doses (5.1)
- May cause serotonin syndrome when used with antidepressants (5.2)
- May cause falling asleep during activities of daily living, daytime drowsiness, and somnolence (5.3)
- May cause hypotension, especially orthostatic (5.6)
- May cause or exacerbate dyskinesia. Decreasing the levodopa dose may lessen or eliminate this side effect (5.7)
- May cause hallucinations and psychotic-like behavior (5.8)
- May cause impulse control/compulsive behaviors (5.9)
- May cause withdrawal-emergent hyperpyrexia and confusion (5.10)
Adverse Reactions Section
6 ADVERSE REACTIONS
The following adverse reactions are described in more detail in the Warnings and Precautions section of the label:
- Hypertension [see Warnings and Precautions (5.1)]
- Serotonin Syndrome [see Warnings and Precautions (5.2)]
- Falling Asleep During Activities of Daily Living and Somnolence [see Warnings and Precautions (5.3)]
- Hypotension / Orthostatic Hypotension [see Warnings and Precautions (5.6)]
- Dyskinesia [see Warnings and Precautions (5.7)]
- Hallucinations / Psychotic-Like Behavior [see Warnings and Precautions (5.8)]
- Impulse Control /Compulsive Behaviors [see Warnings and Precautions (5.9)]
- Withdrawal-Emergent Hyperpyrexia and Confusion [see Warnings and Precautions (5.10)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to the incidence of adverse reactions in the clinical trials of another drug and may not reflect the rates of adverse reactions observed in practice.
During the clinical development of rasagiline, Parkinson’s disease patients
received rasagiline as initial monotherapy (Study 1) and as adjunct therapy
(Study 2, Study 3, Study 4). As the populations in these studies differ, not
only in the adjunct use of dopamine agonists or levodopa during rasagiline
treatment, but also in the severity and duration of their disease, the adverse
reactions are presented separately for each study.
Monotherapy Use of Rasagiline
In Study 1, approximately 5% of the 149 patients treated with rasagiline
discontinued treatment due to adverse reactions compared to 2% of the 151
patients who received placebo.
The only adverse reaction that led to the discontinuation of more than one
patient was hallucinations.
The most commonly observed adverse reactions in Study 1 (incidence in
rasagiline -treated patients 3% or greater than the incidence in placebo-
treated patients) included flu syndrome, arthralgia, depression, and
dyspepsia. Table 1 lists adverse reactions that occurred in 2% or greater of
patients receiving rasagiline as monotherapy and were numerically more
frequent than in the placebo group in Study 1.
Table 1: Adverse Reactions in Study 1*
|
Rasagiline 1 mg (N=149) |
Placebo (N=151) | |
|
% of Patients |
% of Patients | |
|
Headache |
14 |
12 |
|
Arthralgia |
7 |
4 |
|
Dyspepsia |
7 |
4 |
|
Depression |
5 |
2 |
|
Fall |
5 |
3 |
|
Flu syndrome |
5 |
1 |
|
Conjunctivitis |
3 |
1 |
|
Fever |
3 |
1 |
|
Gastroenteritis |
3 |
1 |
|
Rhinitis |
3 |
1 |
|
Arthritis |
2 |
1 |
|
Ecchymosis |
2 |
0 |
|
Malaise |
2 |
0 |
|
Neck Pain |
2 |
0 |
|
Paresthesia |
2 |
1 |
|
Vertigo |
2 |
1 |
*Incidence 2% or greater in rasagiline 1 mg group and numerically more frequent than in placebo group
There were no significant differences in the safety profile based on age or gender.
Adjunct Use of Rasagiline
Rasagiline was studied as an adjunct therapy without levodopa (Study 2), or as an adjunct therapy to levodopa, with some patients also taking dopamine agonists, COMT inhibitors, anticholinergics, or amantadine (Study 3 and Study 4).
In Study 2, approximately 8% of the 162 patients treated with rasagiline discontinued treatment due to adverse reactions compared to 4% of the 164 patients who received placebo.
Adverse reactions that led to the discontinuation of more than one patient were nausea and dizziness.
The most commonly observed adverse reactions in Study 2 (incidence in
rasagiline -treated patients 3% or greater than incidence in placebo-treated
patients) included peripheral edema, fall, arthralgia, cough, and insomnia.
Table 2 lists adverse reactions that occurred in 2% or greater in patients
receiving rasagiline as adjunct therapy without levodopa and numerically more
frequent than in the placebo group in Study 2.
Table 2: Adverse Reactions in Study 2*
|
Rasagiline 1 mg (N=162) |
Placebo (N=164) | |
|
% of Patients |
% of Patients | |
|
Dizziness |
7 |
6 |
|
Peripheral edema |
7 |
4 |
|
Headache |
6 |
4 |
|
Nausea |
6 |
4 |
|
Fall |
6 |
1 |
|
Arthralgia |
5 |
2 |
|
Back pain |
4 |
3 |
|
Cough |
4 |
1 |
|
Insomnia |
4 |
1 |
|
Upper respiratory tract infection |
4 |
2 |
|
Orthostatic hypotension |
3 |
1 |
*Incidence 2% or greater in rasagiline 1 mg group and numerically more frequent than in placebo group
There were no significant differences in the safety profile based on age or gender.
In Study 3, adverse event reporting was considered more reliable than Study 4; therefore, only the adverse event data from Study 3 are presented below.
In Study 3, approximately 9% of the 164 patients treated with rasagiline 0.5
mg/day and 7% of the 149 patients treated with rasagiline 1 mg/day
discontinued treatment due to adverse reactions, compared to 6% of the 159
patients who received placebo. The adverse reactions that led to
discontinuation of more than one rasagiline -treated patient were diarrhea,
weight loss, hallucination, and rash.
The most commonly observed adverse reactions in Study 3 (incidence in
rasagiline -treated patients 3% or greater than the incidence in placebo-
treated patients) included dyskinesia, accidental injury, weight loss,
postural hypotension, vomiting, anorexia, arthralgia, abdominal pain, nausea,
constipation, dry mouth, rash, abnormal dreams, fall and tenosynovitis.
Table 3 lists adverse reactions that occurred in 2% or greater of patients
treated with rasagiline 1 mg/day and that were numerically more frequent than
the placebo group in Study 3.
Table 3: Adverse Reactions in Study 3*
|
Rasagiline 1 mg (N=149) |
Rasagiline 0.5 mg (N=164) |
Placebo | |
|
% of patients |
% of patients |
% of patients | |
|
Dyskinesia |
18 |
18 |
10 |
|
Accidental injury |
12 |
8 |
5 |
|
Nausea |
12 |
10 |
8 |
|
Headache |
11 |
8 |
10 |
|
Fall |
11 |
12 |
8 |
|
Weight loss |
9 |
2 |
3 |
|
Constipation |
9 |
4 |
5 |
|
Postural hypotension |
9 |
6 |
3 |
|
Arthralgia |
8 |
6 |
4 |
|
Vomiting |
7 |
4 |
1 |
|
Dry mouth |
6 |
2 |
3 |
|
Rash |
6 |
3 |
3 |
|
Somnolence |
6 |
4 |
4 |
|
Abdominal pain |
5 |
2 |
1 |
|
Anorexia |
5 |
2 |
1 |
|
Diarrhea |
5 |
7 |
4 |
|
Ecchymosis |
5 |
2 |
3 |
|
Dyspepsia |
5 |
4 |
4 |
|
Paresthesia |
5 |
2 |
3 |
|
Abnormal dreams |
4 |
1 |
1 |
|
Hallucinations |
4 |
5 |
3 |
|
Ataxia |
3 |
6 |
1 |
|
Dyspnea |
3 |
5 |
2 |
|
Infection |
3 |
2 |
2 |
|
Neck pain |
3 |
1 |
1 |
|
Sweating |
3 |
2 |
1 |
|
Tenosynovitis |
3 |
1 |
0 |
|
Dystonia |
3 |
2 |
1 |
|
Gingivitis |
2 |
1 |
1 |
|
Hemorrhage |
2 |
1 |
1 |
|
Hernia |
2 |
1 |
1 |
|
Myasthenia |
2 |
2 |
1 |
*Incidence 2% or greater in rasagiline 1 mg group and numerically more frequent than in placebo group
Several of the more common adverse reactions seemed dose-related, including weight loss, postural hypotension, and dry mouth.
There were no significant differences in the safety profile based on age or gender.
During all Parkinson’s disease phase 2/3 clinical trials, the long-term safety profile was similar to that observed with shorter duration exposure.
6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use
of rasagiline. Because these reactions are reported voluntarily from a
population of uncertain size, it is not always possible to reliably estimate
their frequency or establish a causal relationship to drug exposure.
Skin and Subcutaneous Tissue Disorders: Melanoma
Most common adverse reactions (incidence 3% or greater than placebo):
- Rasagiline monotherapy: flu syndrome, arthralgia, depression, dyspepsia (6.1)
- Rasagiline used as adjunct without levodopa: peripheral edema, fall, arthralgia, cough, and insomnia (6.1)
- Rasagiline used as adjunct to levodopa: dyskinesia, accidental injury, weight loss, postural hypotension, vomiting, anorexia, arthralgia, abdominal pain, nausea, constipation, dry mouth, rash, abnormal dreams, fall, and tenosynovitis (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Ascend Laboratories, LLC at 1-877-ASC-RX01 (877-272-7901) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions Section
7 DRUG INTERACTIONS
7.1 Meperidine
Serious, sometimes fatal reactions have been precipitated with concomitant use of meperidine (e.g., Demerol and other tradenames) and MAO inhibitors including selective MAO-B inhibitors [see Contraindications (4)].
7.2 Dextromethorphan
The concomitant use of rasagiline and dextromethorphan was not allowed in clinical studies. The combination of MAO inhibitors and dextromethorphan has been reported to cause brief episodes of psychosis or bizarre behavior. Therefore, in view of rasagiline’s MAO inhibitory activity, dextromethorphan is contraindicated for use with rasagiline [see Contraindications (4)].
7.3 MAO Inhibitors
Rasagiline is contraindicated for use with other MAO inhibitors because of the increased risk of nonselective MAO inhibition that may lead to a hypertensive crisis [see Contraindications (4)].
7.4 Sympathomimetic Medications
The concomitant use of rasagiline and sympathomimetic medications was not
allowed in clinical studies. Severe hypertensive reactions have followed the
administration of sympathomimetics and nonselective MAO inhibitors.
Hypertensive crisis has been reported in patients taking the recommended dose
of rasagiline and sympathomimetic medications. Severe hypertension has been
reported in patients taking the recommended dose of rasagiline and ophthalmic
drops containing sympathomimetic medications.
Because rasagiline is a selective MAOI, hypertensive reactions are not
ordinarily expected with the concomitant use of sympathomimetic medications.
Nevertheless, caution should be exercised when concomitantly using recommended
doses of rasagiline with any sympathomimetic medications including nasal,
oral, and ophthalmic decongestants and cold remedies.
7.5 Antidepressants
Concomitant use of rasagiline with one of many classes of antidepressants (e.g., SSRIs, SNRIs, triazolopyridine, tricyclic or tetracyclic antidepressants) is not recommended [see Warnings and Precautions (5.2) and Clinical Pharmacology (12.3)]. Concomitant use of rasagiline and MAO inhibitors is contraindicated [see Contraindications (4)].
7.6 Ciprofloxacin or Other CYP1A2 Inhibitors
Rasagiline plasma concentrations may increase up to 2 fold in patients using concomitant ciprofloxacin and other CYP1A2 inhibitors. This could result in increased adverse events. Patients taking concomitant ciprofloxacin or other CYP1A2 inhibitors should not exceed a dose of rasagiline 0.5 mg once daily [see Warnings and Precautions (5.4) and Clinical Pharmacology (12.3)].
7.7 Tyramine/Rasagiline Interaction
MAO in the gastrointestinal tract and liver (primarily type A) provides
protection from exogenous amines (e.g., tyramine) that have the capacity, if
absorbed intact, to cause a tyramine reaction with hypertension including
clinical syndromes referred to as hypertensive urgency, crisis, or emergency.
Foods and medications containing large amounts of exogenous amines (e.g., from
fermented cheese, herring, over-the-counter cough/cold medications) may cause
release of norepinephrine resulting in a rise in systemic blood pressure.
Results of a special tyramine challenge study indicate that rasagiline is
selective for MAO-B at recommended doses and can be used without dietary
tyramine restriction. However, certain foods may contain very high amounts
(i.e., 150 mg or greater) of tyramine and could potentially cause a
hypertensive reaction in individual patients taking rasagiline due to
increased sensitivity to tyramine. Selectivity for inhibiting MAO-B diminishes
in a dose-related manner as the dose is progressively increased above the
recommended daily doses.
There were no cases of hypertensive crisis in the clinical development program
associated with 1 mg daily rasagiline treatment, in which most patients did
not follow dietary tyramine restriction.
There have been postmarketing reports of patients who experienced
significantly elevated blood pressure (including rare cases of hypertensive
crisis) after ingestion of unknown amounts of tyramine-rich foods while taking
recommended doses of rasagiline. Patients should be advised to avoid foods
containing a very large amount of tyramine while taking recommended doses of
rasagiline [see Warnings and Precautions (5.1)].
7.8 Dopaminergic Antagonists
It is possible that dopamine antagonists, such as antipsychotics or metoclopramide, could diminish the effectiveness of rasagiline.
- Meperidine: Risk of serotonin syndrome (4, 7.1)
- Dextromethorphan: Risk of psychosis or bizarre behavior (4, 7.2)
- MAO inhibitors: Risk of non-selective MAO inhibition and hypertensive crisis (4, 7.3)
Dosage & Administration Section
2 DOSAGE AND ADMINISTRATION
2.1 General Dosing Recommendations
When rasagiline tablets are prescribed as monotherapy or as adjunct therapy in patients not taking levodopa, patients may start rasagiline at the recommended dose of 1 mg administered orally once daily.
In patients taking levodopa, with or without other PD drugs (e.g., dopamine agonist, amantadine, anticholinergics), the recommended initial dose of rasagiline tablets are 0.5 mg once daily. If the patient tolerates the daily 0.5 mg dose, but a sufficient clinical response is not achieved, the dose may be increased to 1 mg once daily. When rasagiline is used in combination with levodopa, a reduction of the levodopa dose may be considered, based upon individual response.
The recommended doses of rasagiline should not be exceeded because of risk of hypertension [See Warnings and Precautions (5.1)].
2.2 Patients Taking Ciprofloxacin or Other CYP1A2 Inhibitors
Patients taking concomitant ciprofloxacin or other CYP1A2 inhibitors should not exceed a dose of rasagiline 0.5 mg once daily [see Warnings and Precautions (5.4), Drug Interactions (7.6), and Clinical Pharmacology (12.3)].
2.3 Patients with Hepatic Impairment
Patients with mild hepatic impairment should not exceed a dose of rasagiline 0.5 mg once daily. Rasagiline should not be used in patients with moderate or severe hepatic impairment [see Warnings and Precautions (5.5), Use in Specific Populations (8.6), and Clinical Pharmacology (12.3)].
- Monotherapy: Rasagiline tablets 1 mg once daily (2.1)
- As adjunct without levodopa: Rasagiline tablets 1 mg once daily (2.1)
- As adjunct to levodopa: Rasagiline tablets 0.5 mg once daily. Increase dose to 1 mg daily as needed for sufficient clinical response (2.1)
- Patients taking ciprofloxacin or other CYP1A2 inhibitors: Rasagiline tablets 0.5 mg once daily (2.2, 5.4)
- Patients with mild hepatic impairment: Rasagiline tablets 0.5 mg once daily. Rasagiline tablets should not be used in patients with moderate or severe hepatic impairment (2.3, 5.5)
Dosage Forms & Strengths Section
3 DOSAGE FORMS AND STRENGTHS
Rasagiline tablets 0.5 mg: White to off white, uncoated round, flat, beveled tablets debossed with "RAS" on one side and "0.5" on other side containing, as the active ingredient, rasagiline mesylate equivalent to 0.5 mg of rasagiline base.
Rasagiline tablets 1 mg: White to off white, uncoated round, flat, beveled tablets debossed with "RAS" on one side and "1" on other side containing, as the active ingredient, rasagiline mesylate equivalent to 1 mg of rasagiline base.
· Rasagiline tablets 0.5 mg tablets (3)
· Rasagiline tablets 1 mg tablets (3) (3)
Use In Specific Populations Section
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no adequate data on the developmental risks associated with the use of rasagiline in pregnant women. In animal studies, oral administration of rasagiline to rats during gestation and lactation resulted in decreased survival and reduced body weight in the offspring at doses similar to those used clinically. When administered to pregnant animals in combination with levodopa/carbidopa, there were increased incidences of fetal skeletal variations in rats and increases in embryofetal death and cardiovascular abnormalities in rabbits [see Data].
In the US general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively. The background risks of major birth defects and miscarriage for the indicated population is unknown.
Data
Animal Data
In a combined mating/fertility and embryofetal development study in pregnant rats, no effect on embryofetal development was observed at oral doses up to 3 mg/kg/day (approximately 30 times the plasma exposure (AUC) in humans at the maximum recommended human dose [MRHD, 1mg/day]).
In pregnant rabbits administered rasagiline throughout the period of organogenesis at oral doses of up to 36 mg/kg/day, no developmental toxicity was observed. At the highest dose tested, the plasma AUC was approximately 800 times that in humans at the MRHD.
In pregnant rats administered rasagiline (0, 0.1, 0.3, 1 mg/kg/day) orally during gestation and lactation, offspring survival was decreased and offspring body weight was reduced at 0.3 mg/kg/day and 1 mg/kg/day (10 and 16 times the plasma AUC in humans at the MRHD). The no-effect dose (0.1 mg/kg) for adverse developmental effects is similar to the MRHD on a body surface area (mg/m2) basis. The effect of rasagiline on physical and behavioral development was not adequately assessed in this study.
Rasagiline may be given as an adjunct therapy to levodopa/carbidopa treatment. In pregnant rats administered rasagiline (0, 0.1, 0.3, 1 mg/kg/day) and levodopa/carbidopa (80/20 mg/kg/day) (alone and in combination) orally throughout the period of organogenesis, there was an increased incidence of fetal skeletal variations in fetuses from rats treated with rasagiline in combination with levodopa/carbidopa at 1/80/20 mg/kg/day (approximately 8 times the rasagiline plasma AUC in humans at the MRHD and similar to the MRHD of levodopa/carbidopa [800/200 mg/day] on a mg/m2 basis). In pregnant rabbits dosed orally throughout the period of organogenesis with rasagiline alone (3 mg/kg) or in combination with levodopa/carbidopa (rasagiline: 0.1, 0.6, 1.2 mg/kg, levodopa/carbidopa: 80/20 mg/kg/day), an increase in embryofetal death was noted at rasagiline doses of 0.6 and 1.2 mg/kg/day when administered in combination with levodopa/carbidopa (approximately 7 and 13 times, respectively, the rasagiline plasma AUC in humans at the MRHD). There was an increase in cardiovascular abnormalities with levodopa/carbidopa alone (similar to the MRHD on a mg/m2 basis) and to a greater extent when rasagiline (at all doses; 1-13 times the rasagiline plasma AUC in humans at the MRHD) was administered in combination with levodopa/carbidopa.
8.2 Lactation
Risk Summary
There are no data on the presence of rasagiline in human milk or the effects on the breastfed infant. In rats, rasagiline was shown to inhibit prolactin secretion. The clinical relevance in humans is unknown, and there are no data on the effects of rasagiline on prolactin secretion or milk production in humans.
The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for rasagiline and any potential adverse effects on the breastfed infant from rasagiline or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness in pediatric patients have not been established.
8.5 Geriatric Use
Approximately half of patients in clinical trials were 65 years and over. There were no significant differences in the safety profile of the geriatric and nongeriatric patients.
8.6 Hepatic Impairment
Rasagiline plasma concentration may be increased in patients with mild (up to 2 fold, Child-Pugh score 5 to 6), moderate (up to 7 fold, Child-Pugh score 7 to 9), and severe (Child-Pugh score 10 to 15) hepatic impairment. Patients with mild hepatic impairment should not exceed a dose of 0.5 mg/day. Rasagiline should not be used in patients with moderate or severe hepatic impairment [see Dosage and Administration (2.3), Warnings and Precautions (5.5) and Clinical Pharmacology (12.3)].
8.7 Renal Impairment
Dose adjustment of rasagiline is not required for patients with mild or moderate renal impairment because rasagiline plasma concentrations are not increased in patients with moderate renal impairment. Rasagiline has not been studied in patients with severe renal impairment [see Clinical Pharmacology (12.3)].
- Pregnancy: Based on animal data, may cause fetal harm. (8.1)
Drug Abuse And Dependence Section
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
Rasagiline tablets are not a controlled substance.
9.2 Abuse
Studies conducted in mice and rats did not reveal any potential for drug abuse and dependence. Clinical trials have not revealed any evidence of the potential for abuse, tolerance or physical dependence; however, systematic studies in humans designed to evaluate these effects have not been performed.
9.3 Dependence
Studies conducted in mice and rats did not reveal any potential for drug abuse and dependence. Clinical trials have not revealed any evidence of the potential for abuse, tolerance or physical dependence; however, systematic studies in humans designed to evaluate these effects have not been performed.
Overdosage Section
10 OVERDOSAGE
In a dose escalation study in patients on chronic levodopa therapy treated
with 10 mg of rasagiline there were three reports of cardiovascular side
effects (including hypertension and postural hypotension) which resolved
following treatment discontinuation.
Although no cases of overdose have been observed with rasagiline during the
clinical development program, the following description of presenting symptoms
and clinical course is based upon overdose descriptions of nonselective MAO
inhibitors.
The signs and symptoms of nonselective MAOI overdose may not appear
immediately. Delays of up to 12 hours after ingestion of drug and the
appearance of signs may occur. The peak intensity of the syndrome may not be
reached until for a day following the overdose. Death has been reported
following overdose; therefore, immediate hospitalization, with continuous
patient observation and monitoring for at least two days following the
ingestion of such drugs in overdose, is strongly recommended.
The severity of the clinical signs and symptoms of MAOI overdose varies and
may be related to the amount of drug consumed. The central nervous and
cardiovascular systems are prominently involved.
Signs and symptoms of MAOI overdose may include: drowsiness, dizziness,
faintness, irritability, hyperactivity, agitation, severe headache,
hallucinations, trismus, opisthotonos, convulsions, and coma; rapid and
irregular pulse, hypertension, hypotension and vascular collapse; precordial
pain, respiratory depression and failure, hyperpyrexia, diaphoresis, and cool,
clammy skin.
There is no specific antidote for rasagiline overdose. The following
suggestions are offered based upon the assumption that rasagiline overdose may
be modeled after nonselective MAO inhibitor poisoning. Treatment of overdose
with nonselective MAO inhibitors is symptomatic and supportive. Respiration
should be supported by appropriate measures, including management of the
airway, use of supplemental oxygen, and mechanical ventilatory assistance, as
required. Body temperature should be monitored closely. Intensive management
of hyperpyrexia may be required. Maintenance of fluid and electrolyte balance
is essential. For this reason, in cases of overdose with rasagiline, dietary
tyramine restriction should be observed for several weeks to reduce the risk
of hypertensive tyramine reaction.
A poison control center should be called for the most current treatment
guidelines.
A postmarketing report described a single patient who developed a nonfatal
serotonin syndrome after ingesting 100 mg of rasagiline in a suicide attempt.
Another patient who was treated in error with 4 mg rasagiline daily and
tramadol also developed a serotonin syndrome. One patient who was treated in
error with 3 mg rasagiline daily experienced alternating episodes of vascular
fluctuations consisting of hypertension and orthostatic hypotension.
Description Section
11 DESCRIPTION
Rasagiline tablets contain rasagiline (as the mesylate), a propargylamine- based drug indicated for the treatment of idiopathic Parkinson’s disease. Rasagiline mesylate is designated chemically as: 1H-Inden-1-amine, 2, 3dihydro-N-2-propynyl-, (1R)-, methane sulfonate. The empirical formula of rasagiline mesylate is (C12H13NCH4SO3 and its molecular weight is 267.34.
Its structural formula is:
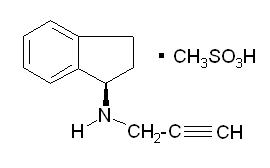
Rasagiline mesylate is a white to off-white powder, freely soluble in water or
ethanol and sparingly soluble in isopropanol. Each rasagiline tablets for oral
administration contains 0.5mg or 1mg of rasagiline (equivalent to 0.78 mg or
1.56 mg of rasagiline mesylate).
Each rasagiline tablet also contains the following inactive ingredients:
microcrystalline cellulose, corn starch, colloidal silicon dioxide, citric
acid monohydrate, pregelatinized starch, stearic acid and talc.
Clinical Pharmacology Section
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Rasagiline is a selective, irreversible MAO-B inhibitor indicated for the
treatment of idiopathic Parkinson’s disease. The results of a clinical trial
designed to examine the effects of rasagiline on blood pressure when it is
administered with increasing doses of tyramine indicates the functional
selectivity can be incomplete when healthy subjects ingest large amounts of
tyramine while receiving recommended doses of rasagiline. The selectivity for
inhibiting MAO-B diminishes in a dose-related manner.
MAO, a flavin-containing enzyme, is classified into two major molecular
species, A and B, and is localized in mitochondrial membranes throughout the
body in nerve terminals, brain, liver and intestinal mucosa. MAO regulates the
metabolic degradation of catecholamines and serotonin in the CNS and
peripheral tissues. MAO-B is the major form in the human brain. In ex vivo
animal studies in brain, liver, and intestinal tissues, rasagiline was shown
to be a potent, irreversible monoamine oxidase type B (MAO-B) selective
inhibitor. Rasagiline at the recommended therapeutic dose was also shown to be
a potent and irreversible inhibitor of MAO-B in platelets. The precise
mechanisms of action of rasagiline are unknown. One mechanism is believed to
be related to its MAO-B inhibitory activity, which causes an increase in
extracellular levels of dopamine in the striatum. The elevated dopamine level
and subsequent increased dopaminergic activity are likely to mediate
rasagiline’s beneficial effects seen in models of dopaminergic motor
dysfunction.
12.2 Pharmacodynamics
Tyramine Challenge Test
Results of a tyramine challenge study indicate that rasagiline at recommended
doses is relatively selective for inhibiting MAO-B and can be used without
dietary tyramine restriction. However, certain foods (e.g., aged cheeses, such
as Stilton cheese) may contain very high amounts of tyramine (i.e., 150 mg or
greater) and could potentially cause severe hypertension caused by tyramine
interaction in patients taking rasagiline due to mild increased sensitivity to
tyramine at recommended doses. Relative selectivity of rasagiline for
inhibiting MAO-B diminished in a dose-related manner as the dose progressively
increased above the highest recommended daily dose (1 mg) [see Warnings and Precautions (5.1) and Drug Interactions (7.7)].
Platelet MAO Activity in Clinical Studies
Studies in healthy subjects and in Parkinson’s disease patients have shown
that rasagiline inhibits platelet MAO-B irreversibly. The inhibition lasts at
least 1 week after last dose. Almost 25to35% MAO-B inhibition was achieved
after a single rasagiline dose of 1 mg/day and more than 55% of MAO-B
inhibition was achieved after a single rasagiline dose of 2 mg/day. Over 90%
inhibition was achieved 3 days after rasagiline daily dosing at 2 mg/day and
this inhibition level was maintained 3 days postdose. Multiple doses of
rasagiline of 0.5, 1 and 2 mg per day resulted in complete MAO-B inhibition.
12.3 Pharmacokinetics
Rasagiline in the range of 1 to 6 mg demonstrated a more than proportional increase in AUC, while Cmax was dose proportional. Rasagiline mean steady- state half life is 3 hours but there is no correlation of pharmacokinetics with its pharmacological effect because of its irreversible inhibition of MAO-B.
Absorption
Rasagiline is rapidly absorbed, reaching peak plasma concentration (Cmax) in
approximately 1 hour. The absolute bioavailability of rasagiline is about 36%.
Food does not affect the Tmax of rasagiline, although Cmax and exposure (AUC)
are decreased by approximately 60% and 20%, respectively, when the drug is
taken with a high fat meal. Because AUC is not significantly affected,
rasagiline can be administered with or without food.
Distribution
The mean volume of distribution at steady-state is 87 L, indicating that the
tissue binding of rasagiline is in excess of plasma protein binding. Plasma
protein binding ranges from 88 to 94% with mean extent of binding of 61 to 63%
to human albumin over the concentration range of 1 to 100 ng/mL.
Metabolism and Elimination
Rasagiline undergoes almost complete biotransformation in the liver prior to
excretion. The metabolism of rasagiline proceeds through two main pathways:
N-dealkylation and/or hydroxylation to yield 1-aminoindan (AI), 3-hydroxy-N-
propargyl-1 aminoindan (3-OH-PAI) and 3-hydroxy-1-aminoindan (3-OH-AI). In
vitro experiments indicate that both routes of rasagiline metabolism are
dependent on the cytochrome P450 (CYP) system, with CYP1A2 being the major
isoenzyme involved in rasagiline metabolism. Glucuronide conjugation of
rasagiline and its metabolites, with subsequent urinary excretion, is the
major elimination pathway.
After oral administration of 14C-labeled rasagiline, elimination occurred
primarily via urine and secondarily via feces (62% of total dose in urine and
7% of total dose in feces over 7 days), with a total calculated recovery of
84% of the dose over a period of 38 days. Less than 1% of rasagiline was
excreted as unchanged drug in urine.
Specific Populations
Hepatic Impairment
Following repeat dose administration (7 days) of rasagiline (1 mg/day) in
subjects with mild hepatic impairment (Child-Pugh score 5-6), AUC and Cmax
were increased by 2 fold and 1.4 fold, respectively, compared to healthy
subjects. In subjects with moderate hepatic impairment (Child to Pugh score 7
to 9), AUC and Cmax were increased by 7 fold and 2 fold, respectively,
compared to healthy subjects [see Dosage and Administration (2.3) and Warnings and Precautions (5.5)].
Renal Impairment
Following repeat dose administration (8 days) of rasagiline (1 mg/day) in
subjects with moderate renal impairment, rasagiline exposure (AUC) was similar
to rasagiline exposure in healthy subjects, while the major metabolite 1-AI
exposure (AUC) was increased 1.5-fold in subjects with moderate renal
impairment, compared to healthy subjects. Because 1-AI is not an MAO
inhibitor, no dose adjustment is needed for patients with mild and moderate
renal impairment. Data are not available for patients with severe renal
impairment.
Elderly
Since age has little influence on rasagiline pharmacokinetics, it can be
administered at the recommended dose in the elderly (≥65 years).
Pediatric
Rasagiline has not been investigated in patients below 18 years of age.
Gender
The pharmacokinetic profile of rasagiline is similar in men and women.
Drug-Drug Interactions
Levodopa
A study in Parkinson’s disease patients, in which the effect of
levodopa/carbidopa (LD/CD) on rasagiline pharmacokinetics at steady state was
investigated, showed that the pharmacokinetics of rasagiline were not affected
by concomitant administration of LD/CD.
Effect of Other Drugs on the Metabolism of Rasagiline
In vitro metabolism studies showed that CYP1A2 was the major enzyme
responsible for the metabolism of rasagiline. There is the potential for
inhibitors of this enzyme to alter rasagiline clearance when coadministered
[see Dosage and Administration (2.2) and Warnings and Precautions (5.4)].
Ciprofloxacin: When ciprofloxacin, an inhibitor of CYP1A2, was administered to
healthy volunteers (n=12) at 500 mg (BID) with rasagiline at 2 mg/day, the AUC
of rasagiline increased by 83% and there was no change in the elimination half
life [see Dosage and Administration (2.2) and Warnings and Precautions (5.4)].
Theophylline: Coadministration of rasagiline 1 mg/day and theophylline, a
substrate of CYP1A2, up to 500 mg twice daily to healthy subjects (n=24) did
not affect the pharmacokinetics of either drug.
Antidepressants: Severe CNS toxicity (occasionally fatal) associated with
hyperpyrexia as part of a serotonin syndrome, has been reported with combined
treatment of an antidepressant (e.g., from one of many classes including
tricyclic or tetracyclic antidepressants, SSRIs, SNRIs, triazolopyridine
antidepressants) and nonselective MAOI or a selective MAO-B inhibitor [see Warnings and Precautions (5.2)].
Effect of Rasagiline on Other Drugs
No additional in vivo trials have investigated the effect of rasagiline on
other drugs metabolized by the cytochrome P450 enzyme system. In vitro studies
showed that rasagiline at a concentration of 1 mcg/mL (equivalent to a level
that is 160 times the average Cmax ~ 5.9 to 8.5 ng/mL in Parkinson’s disease
patients after 1 mg rasagiline multiple dosing) did not inhibit cytochrome
P450 isoenzymes, CYP1A2, CYP2A6, CYP2C9, CYP2C19, CYP2D6, CYP2E1, CYP3A4 and
CYP4A. These results indicate that rasagiline is unlikely to cause any
clinically significant interference with substrates of these enzyme.
Nonclinical Toxicology Section
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis & Mutagenesis & Impairment of Fertility
Carcinogenesis
Two-year carcinogenicity studies were conducted in mice at oral doses of 0,1, 15, and 45 mg/kg/day and in rats at oral doses of 0.3, 1, and 3 mg/kg/day (males) or 0, 0.5, 2, 5, and 17 mg/kg/day (females). In rats, there was no increase in tumors at any dose tested. Plasma exposures (AUC) at the highest dose tested were approximately 33 and 260 times, in male and female rats, respectively, that in humans at the maximum recommended human dose (MRHD) of 1 mg/day.
In mice, there was an increase in lung tumors (combined adenomas/carcinomas) at 15 and 45 mg/kg in males and females. At the lowest dose tested, plasma AUCs were approximately 5 times those expected in humans at the MRHD.
The carcinogenic potential of rasagiline administered in combination with levodopa/carbidopa has not been examined.
Mutagenesis
Rasagiline was reproducibly clastogenic in in vitro chromosomal aberration assays in human lymphocytes in the presence of metabolic activation and was mutagenic and clastogenic in the in vitro mouse lymphoma tk assay in the absence and presence of metabolic activation. Rasagiline was negative in the in vitro bacterial reverse mutation (Ames) assay and in the in vivo micronucleus assay in mice. Rasagiline was also negative in the in vivo micronucleus assay in mice when administered in combination with levodopa/carbidopa.
Impairment of Fertility
Rasagiline had no effect on mating performance or fertility in rats treated prior to and throughout the mating period and continuing in females through gestation day 17 at oral doses of up to 3 mg/kg/day (approximately 30 times the plasma AUC in humans at the MRHD). The effect of rasagiline administered in combination with levodopa/carbidopa on mating and fertility has not been examined.
Clinical Studies Section
14 CLINICAL STUDIES
The effectiveness of rasagiline for the treatment of Parkinson’s disease was established in four 18 to 26-week, randomized, placebo-controlled trials, as initial monotherapy or adjunct therapy.
14.1 Monotherapy Use of Rasagiline
Study 1 was a double-blind, randomized, fixed-dose parallel group, 26-week
study in early Parkinson’s disease patients not receiving any concomitant
dopaminergic therapy at the start of the study. The majority of the patients
were not treated with medications for Parkinson’s disease before receiving
rasagiline.
In Study 1, 404 patients were randomly assigned to receive placebo (138
patients), rasagiline 1 mg/day (134 patients) or rasagiline 2 mg/day (132
patients). Patients were not allowed to take levodopa, dopamine agonists,
selegiline or amantadine, but could take stable doses of anticholinergic
medication, if necessary. The average Parkinson’s disease duration was
approximately 1 year (range 0 to 11 years).
The primary measure of effectiveness was the change from baseline in the total
score of the Unified Parkinson’s Disease Rating Scale (UPDRS), [mentation (Part I) + activities of daily living (ADL) (Part II) + motor function (Part III)]. The UPDRS is a multi-item rating scale that measures the ability of a
patient to perform mental and motor tasks as well as activities of daily
living. A reduction in the score represents improvement and a beneficial
change from baseline appears as a negative number.
Rasagiline (1 or 2 mg once daily) was superior to placebo on the primary
measure of effectiveness in patients receiving six months of treatment and not
on dopaminergic therapy. The effectiveness of rasagiline 1 mg and 2 mg was
comparable. Table 4 shows the results of Study 1. There were no differences in
effectiveness based on age or gender between rasagiline 1 mg/day and placebo
** Table 4: Change in Total UPDRS Score in Study 1**
|
Baseline score |
Change from baseline to termination score |
p-value vs. placebo | |
|
Placebo |
24.5 |
3.9 |
-- |
|
Rasagiline 1 mg |
24.7 |
0.1 |
0.0001 |
|
Rasagiline 2 mg |
25.9 |
0.7 |
0.0001 |
14.2 Adjunct Use of Rasagiline
Study 2 was a double-blind, randomized, placebo-controlled, parallel group,
18-week study, investigating rasagiline 1 mg as adjunct therapy to dopamine
agonists without levodopa. Patients were on a stable dose of dopamine agonist
(ropinirole, mean 8 mg/day or pramipexole, mean 1.5 mg/day) therapy for ≥ 30
days, but at doses not sufficient to control Parkinson’s disease symptoms.
In Study 2, 321 patients randomly received placebo (162 patients) or
rasagiline 1 mg/day (159 patients) and had a post-baseline assessment. The
average Parkinson’s disease duration was approximately 2 years (range 0.1 to
14.5 years).
The primary measure of effectiveness was the change from baseline in the total
score of the Unified Parkinson’s Disease Rating Scale (UPDRS) [mentation (Part I) + activities of daily living (ADL) (Part II) + motor function (Part III)].
In Study 2, rasagiline 1 mg was superior to placebo on the primary measure of
effectiveness (see Table 5).****
** Table 5: Change in Total UPDRS Score in Study 2**
|
Baseline score |
Change from baseline to termination score* |
p-value vs. placebo | |
|
Placebo |
29.8 |
–1.2 |
-- |
|
Rasagiline 1 mg |
32.1 |
–3.6 |
0.012 |
*A negative change from baseline indicates improvement in the UPDRS
Secondary outcome assessment of the individual subscales of the UPDRS indicates that the UPDRS Part III motor subscale was primarily responsible for the overall rasagiline effect on the UPDRS score (see Table 6).
** Table 6: Secondary Measures of Effectiveness in Study 2**
|
Baseline (score) |
Change from baseline to termination score | |
|
UPDRS Part II ADL (Activities of Daily Living) subscale score | ||
|
Placebo |
7.9 |
0.4 |
|
Rasagiline 1 mg |
8.6 |
-0.3 |
|
UPDRS Part III Motor subscale score | ||
|
Placebo |
20.4 |
-1.2 |
|
Rasagiline 1 mg |
22.2 |
-3.7 |
Study 3 and Study 4 were randomized, multinational trials conducted in more advanced Parkinson’s disease patients treated chronically with levodopa and experiencing motor fluctuations (including but not limited to, end of dose “wearing off,” sudden or random “off,” etc.). Study 3 was conducted in North America (U.S. and Canada) and compared rasagiline 0.5 mg and 1 mg daily to placebo. Study 4 was conducted outside of North America in Europe, Argentina and Israel, and compared rasagiline 1 mg daily to placebo.
Patients had Parkinson’s disease for an average of 9 years (range 5 months to
33 years), had taken levodopa for an average of 8 years (range 5 months to 32
years), and had motor fluctuations for approximately 3 to 4 years (range 1
month to 23 years). Patients kept home Parkinson’s disease diaries just prior
to baseline and at specified intervals during the trial. Diaries recorded one
of the following four conditions for each half-hour interval over a 24-hour
period: “ON” (period of relatively good function and mobility) as either “ON”
with no dyskinesia or without troublesome dyskinesia, or “ON” with troublesome
dyskinesia, “OFF” (period of relatively poor function and mobility) or asleep.
“Troublesome” dyskinesia is defined as dyskinesia that interferes with the
patient’s daily activity. All patients had inadequate control of their motor
symptoms with motor fluctuations typical of advanced stage disease despite
receiving levodopa/decarboxylase inhibitor. The average dose of levodopa taken
with a decarboxylase inhibitor was approximately 700 to 800 mg (range 150 to
3000 mg/day). Patients continued their stable doses of additional anti-PD
medications at entry into the trials. Approximately 65% of patients in both
studies were also taking a dopamine agonist. In the North American study
(Study 3), approximately 35% of patients took entacapone with
levodopa/decarboxylase inhibitor. The majority of patients taking entacapone
were also taking a dopamine agonist.
In Study 3 and Study 4, the primary measure of effectiveness was the change in
the mean number of hours spent in the “OFF” state at baseline compared to the
mean number of hours spent in the “OFF” state during the treatment period.
In Study 3, patients were randomly assigned to receive placebo (159 patients),
rasagiline 0.5 mg/day (164 patients), or rasagiline 1 mg/day (149 patients)
for 26 weeks. Patients averaged 6 hours daily in the “OFF” state at baseline
as confirmed by home diaries.
In Study 4, patients were randomly assigned to receive placebo (229 patients),
rasagiline 1 mg/day (231 patients) or a COMT inhibitor (active comparator),
taken along with scheduled doses of levodopa/decarboxylase inhibitor (227
patients) for 18 weeks. Patients averaged 5.6 hours daily in the “OFF” state
at baseline as confirmed by home diaries.
In Study 3 and Study 4, rasagiline 1 mg once daily reduced “OFF” time compared
to placebo when added to levodopa in patients experiencing motor fluctuations
(Tables 7 and 8). The lower dose (0.5 mg) of rasagiline also significantly
reduced “OFF” time (Table 7), but had a numerically smaller effect than the 1
mg dose of rasagiline. In Study 4, the active comparator also reduced “OFF”
time when compared to placebo.
Table 7: Change in mean total daily “OFF” time in Study 3
|
Baseline (hours) |
Change from baseline to treatment period (hours) |
p-value vs. placebo | |
|
Placebo |
6.0 |
-0.9 |
-- |
|
Rasagiline 0.5mg |
6.0 |
-1.4 |
0.0199 |
|
Rasagiline 1 mg |
6.3 |
-1.9 |
< 0.0001 |
Table 8 : Change in mean total daily “OFF” time in Study 4
|
Baseline (hours) |
Change from baseline to treatment period (hours) |
p-value vs. placebo | |
|
Placebo |
5.5 |
-0.40 |
-- |
|
Rasagiline 1 mg |
5.6 |
-1.2 |
0.0001 |
In Study 3 and Study 4, dose reduction of levodopa was allowed within the
first 6 weeks, if dopaminergic side effects developed including dyskinesia or
hallucinations. In Study 3, the levodopa dose was reduced in 8% of patients in
the placebo group and in 16% and 17% of patients in the 0.5 mg/day and 1
mg/day rasagiline groups, respectively. When levodopa was reduced, the dose
was reduced by 7%, 9%, and 13% in the placebo, 0.5 mg/day, and 1 mg/day
groups, respectively. In Study 4, levodopa dose reduction occurred in 6% of
patients in the placebo group and in 9% in the rasagiline 1 mg/day groups,
respectively. When levodopa was reduced, it was reduced by 13% and 11% in the
placebo and the rasagiline groups, respectively.
There were no differences in effectiveness based on age or gender between
rasagiline 1 mg/day and placebo.
Several secondary outcome assessments in the two studies showed statistically
significant improvements with rasagiline. These included effects on the
activities of daily living (ADL) subscale of the UPDRS performed during an
“OFF” period and the motor subscale of the UPDRS performed during an “ON”
period. In both scales, a negative response represents improvement. Table 9
and 10 show these results for Studies 3 and 4.
Table 9: Secondary Measures of Effectiveness in Study 3
|
Baseline (score) |
Change from baseline to last value | |
|
UPDRS ADL (Activities of Daily Living) subscale score while “OFF” | ||
|
Placebo |
15.5 |
0.68 |
|
Rasagiline 0.5 mg |
15.8 |
-0.60 |
|
Rasagiline 1 mg |
15.5 |
-0.68 |
|
UPDRS Motor subscale score while “ON” | ||
|
Placebo |
20.8 |
1.21 |
|
Rasagiline 0.5 mg |
21.5 |
-1.43 |
|
Rasagiline 1 mg |
20.9 |
-1.30 |
Table 10: Secondary Measures of Effectiveness in Study 4
|
Baseline (score) |
Change from baseline to last value | |
|
UPDRS ADL (Activities of Daily Living) subscale score while “OFF” | ||
|
Placebo |
18.7 |
-0.89 |
|
Rasagiline 1 mg |
19.0 |
-2.61 |
|
UPDRS Motor subscale score while “ON” | ||
|
Placebo |
23.5 |
-0.82 |
|
Rasagiline 1 mg |
23.8 |
-3.87 |
How Supplied Section
16 HOW SUPPLIED/STORAGE AND HANDLING
Rasagiline Tablets, 0.5 mg
White to off-white, uncoated round, flat, beveled tablets, debossed with “RAS”
on one side and “0.5” on the other side. Supplied as:
NDC 67877-259-30 Bottles of 30 tablets
Rasagiline Tablets, 1 mg
White to off white, uncoated round, flat, beveled tablets debossed with "RAS"
on one side and "1" on other side. Supplied as:
NDC 67877-260-30 Bottles of 30 tablets
Storage:
Store at 25°C (77°F) with excursions permitted to 15° to 30°C (59° to 86°F) [See USP Controlled Room Temperature].
Information For Patients Section
17. PATIENT COUNSELING INFORMATION
Hypertension
Advise patients that treatment with recommended doses of rasagiline tablets
may be associated with elevations of blood pressure. Tell patients who
experience elevation of blood pressure while taking rasagiline tablets to
contact their healthcare provider.
The risk of using higher than recommended daily doses of rasagiline should be
explained, and a brief description of the tyramine associated hypertensive
reaction provided.
Advise patients to avoid certain foods (e.g., aged cheese) containing a very
large amount of tyramine while taking recommended doses of rasagiline tablets
because of the potential for large increases in blood pressure. If patients
eat foods very rich in tyramine and do not feel well soon after eating, they
should contact their healthcare provider [see Warnings and Precautions (5.1)].
Serotonin Syndrome
****Tell patients to inform their physician if they are taking, or planning
to take, any prescription or over-the-counter drugs, especially
antidepressants and over-the-counter cold medications, since there is a
potential for interaction with rasagiline tablets. Because patients should not
use meperidine or certain other analgesics with rasagiline tablets, they
should contact their healthcare provider before taking analgesics [see Contraindications (4) and Warnings and Precautions (5.2)].
Falling Asleep During Activities of Daily Living and Somnolence
Advise and alert patients about the potential for sedating effects associated
with rasagiline tablets and other dopaminergic medications, including
somnolence and particularly to the possibility of falling asleep while engaged
in activities of daily living. Because somnolence can be a frequent adverse
reaction with potentially serious consequences, patients should neither drive
a car nor engage in other potentially dangerous activities until they have
gained sufficient experience with rasagiline tablets and other dopaminergic
medications to gauge whether or not it affects their mental and/or motor
performance adversely. Advise patients that if increased somnolence or new
episodes of falling asleep during activities of daily living (e.g., watching
television, passenger in a car, etc.) are experienced at any time during
treatment, they should not drive or participate in potentially dangerous
activities until they have contacted their physician. Patients should not
drive, operate machinery, or work at heights during treatment if they have
previously experienced somnolence and/or have fallen asleep without warning
prior to use of rasagiline tablets.
Because of possible additive effects, advise patients to exercise caution when
patients are taking other sedating medications, alcohol, or other central
nervous system depressants (e.g., benzodiazepines, antipsychotics,
antidepressants) in combination with rasagiline tablets or when taking
concomitant medications that increase plasma levels of rasagiline (e.g.,
ciprofloxacin) [see Warnings and Precautions (5.3)].
Ciprofloxacin or Other CYP1A2 Inhibitors
Inform patients that they should contact their healthcare provider of
rasagiline tablets if they take ciprofloxacin or a similar drug that could
increase blood levels of rasagiline because of the need to adjust the dose of
rasagiline [see Dosage and Administration (2.2) and Warnings and Precautions (5.4)].
Hepatic Impairment
Tell patients who have hepatic problems to contact their healthcare provider
regarding possible changes in rasagiline tablets dosing [see Warnings and Precautions (5.5)].
Hypotension / Orthostatic Hypotension
Patients should be advised that they may develop orthostatic hypotension with
or without symptoms such as dizziness, nausea, syncope, and sometimes
sweating. Hypotension and/or orthostatic symptoms may occur more frequently
during initial therapy or with an increase in dose at any time (cases have
been seen after weeks of treatment). Accordingly, patients should be cautioned
against standing up rapidly after sitting or lying down, especially if they
have been doing so for prolonged periods, and especially, at the initiation of
treatment with rasagiline tablets [see Warnings and Precautions (5.6)].
Dyskinesia
Advise patients taking rasagiline tablets as adjunct to levodopa that there is
a possibility of dyskinesia or increased dyskinesia [see Warnings and Precautions (5.7)].
Hallucinations / Psychotic-Like Behavior
Inform patients that hallucinations or other manifestations of psychotic-like
behavior can occur when taking rasagiline tablets. Advise patients that, if
they have a major psychotic disorder, that rasagiline should not ordinarily be
used because of the risk of exacerbating the psychosis. Patients with a major
psychotic disorder should also be aware that many treatments for psychosis may
decrease the effectiveness of rasagiline tablets [see Warnings and Precautions (5.8)].
Impulse Control/Compulsive Behaviors
Advise patients that they may experience intense urges to gamble, increased
sexual urges, other intense urges, and the inability to control these urges
while taking one or more of the medications that increase central dopaminergic
tone and that are generally used for the treatment of Parkinson’s disease
(including rasagiline tablets). Although it is not proven that the medications
caused these events, these urges were reported to have stopped in some cases
when the dose was reduced or the medication was stopped. Prescribers should
ask patients about the development of new or increased gambling urges, sexual
urges, or other urges while being treated with rasagiline tablets. Patients
should inform their physician if they experience new or increased gambling
urges, increased sexual urges, or other intense urges while taking rasagiline
tablets. Physicians should consider dose reduction or stopping the medication
if a patient develops such urges while taking rasagiline tablets [see Warnings and Precautions (5.9].
Withdrawal-Emergent Hyperpyrexia and Confusion
Tell patients to contact their healthcare provider if they wish to discontinue
rasagiline tablets [see Warnings and Precautions (5.10)].
Missing Dose
Instruct patients to take rasagiline tablets as prescribed. If a dose is
missed, the patient should not double-up the dose of rasagiline tablets. The
next dose should be taken at the usual time on the following day.
Pregnancy
Advise patients to notify their healthcare provider if they are pregnant or
plan to become pregnant [see Use in Specific Populations (8.1)].
Manufactured by:
Alkem Laboratories Ltd.,
INDIA.
Distributed by:
Ascend Laboratories, LLC
Parsippany, NJ 07054
Revised Date: November, 2021
** PT 1464-04**
