CHLORZOXAZONE
Chlorzoxazone Tablets, USP
52d3aa2c-f88f-4de7-b5ae-df8a46e94879
HUMAN PRESCRIPTION DRUG LABEL
Sep 5, 2023
Aurobindo Pharma Limited
DUNS: 650082092
Products 3
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
CHLORZOXAZONE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (8)
CHLORZOXAZONE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (8)
CHLORZOXAZONE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (10)
Drug Labeling Information
DESCRIPTION SECTION
DESCRIPTION
Each 375 mg chlorzoxazone tablet contains: Chlorzoxazone USP 375 mg.
Each 500 mg chlorzoxazone tablet contains: Chlorzoxazone USP 500 mg.
Each 750 mg chlorzoxazone tablet contains: Chlorzoxazone USP 750 mg.
Chemical Name: 5-Chloro-2-benzoxazolinone.
Structural Formula:
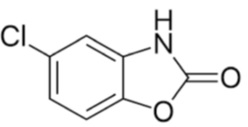
Molecular Formula: C7H4ClNO2
Molecular Weight: 169.56
Chlorzoxazone, USP is a white or practically white, practically odorless, crystalline powder. Chlorzoxazone USP is slightly soluble in water; sparingly soluble in alcohol, in isopropyl alcohol, and in methanol; soluble in solutions of alkali hydroxides and ammonia.
Inactive ingredients:
375 mg contains colloidal silicon dioxide, corn starch, croscarmellose sodium, docusate sodium with sodium benzoate, lactose monohydrate, magnesium stearate and microcrystalline cellulose.
500 mg contains D&C Red 27/Phloxine Aluminium lake, FD&C Yellow 6/Sunset Yellow FCF Aluminium lake, hypromellose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polysorbate 80, pregelatinized starch, sodium starch glycolate.
750 mg contains colloidal silicon dioxide, corn starch, croscarmellose sodium, docusate sodium with sodium benzoate, lactose monohydrate, magnesium stearate and microcrystalline cellulose.
Meets USP dissolution test 5 for 500 mg.
FDA approved dissolution method differs from that of the USP for 375 mg and 750 mg.
