Gabapentin
These highlights do not include all the information needed to use GABAPENTIN TABLETS safely and effectively. See full prescribing information for GABAPENTIN TABLETS. GABAPENTIN tablets, for oral useInitial U.S. Approval: 1993
310ce005-5a24-489a-b23c-d42ab6a3bfed
HUMAN PRESCRIPTION DRUG LABEL
Sep 8, 2025
Zydus Pharmaceuticals USA Inc.
DUNS: 156861945
Products 2
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Gabapentin
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (8)
Gabapentin
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (8)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC 68382-204-01 in bottle of 100 tablets
Gabapentin Tablets USP, 600 mg
Rx only
100 tablets
ZYDUS

NDC 68382-205-01 in bottle of 100 tablets
Gabapentin Tablets USP, 800 mg
Rx only
100 tablets
ZYDUS

ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
The following serious adverse reactions are discussed in greater detail in other sections:
- Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)/Multiorgan Hypersensitivity [see Warnings and Precautions (5.1)]
- Anaphylaxis and Angioedema [see Warnings and Precautions (5.2)]
- Somnolence/Sedation and Dizziness [see Warnings and Precautions (5.4)]
- Suicidal Behavior and Ideation [see Warnings and Precautions (5.5)]
- Increased Risk of Seizures and Other Adverse Reactions with Abrupt or Rapid Discontinuation[see Warnings and Precautions (5.6)]
- Status Epilepticus[see Warnings and Precautions (5.7)]
- Respiratory Depression [see Warnings and Precautions (5.8)]
- Neuropsychiatric Adverse Reactions (Pediatric Patients 3 to 12 Years of Age) [see Warnings and Precautions (5.9)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Postherpetic Neuralgia
The most common adverse reactions associated with the use of gabapentin in adults, not seen at an equivalent frequency among placebo-treated patients, were dizziness, somnolence, and peripheral edema.
In the 2 controlled trials in postherpetic neuralgia, 16% of the 336 patients who received gabapentin and 9% of the 227 patients who received placebo discontinued treatment because of an adverse reaction. The adverse reactions that most frequently led to withdrawal in gabapentin-treated patients were dizziness, somnolence, and nausea.
Table 3 lists adverse reactions that occurred in at least 1% of gabapentin- treated patients with postherpetic neuralgia participating in placebo- controlled trials and that were numerically more frequent in the gabapentin group than in the placebo group.
Table 3 Adverse Reactions in Pooled Placebo-Controlled Trials in Postherpetic Neuralgia|
a Reported as blurred vision | ||
|
** Gabapentin** |
** Placebo** | |
|
** N=336** |
** N=227** | |
|
** %** |
** %** | |
|
** Body as a Whole** | ||
|
Asthenia |
6 |
5 |
|
Infection |
5 |
4 |
|
Accidental injury |
3 |
1 |
|
** Digestive System** | ||
|
Diarrhea |
6 |
3 |
|
Dry mouth |
5 |
1 |
|
Constipation |
4 |
2 |
|
Nausea |
4 |
3 |
|
Vomiting |
3 |
2 |
|
** Metabolic and Nutritional Disorders** | ||
|
Peripheral edema |
8 |
2 |
|
Weight gain |
2 |
0 |
|
Hyperglycemia |
1 |
0 |
|
** Nervous System** | ||
|
Dizziness |
28 |
8 |
|
Somnolence |
21 |
5 |
|
Ataxia |
3 |
0 |
|
Abnormal thinking |
3 |
0 |
|
Abnormal gait |
2 |
0 |
|
Incoordination |
2 |
0 |
|
** Respiratory System** | ||
|
Pharyngitis |
1 |
0 |
|
** Special Senses** | ||
|
Amblyopiaa |
3 |
1 |
|
Conjunctivitis |
1 |
0 |
|
Diplopia |
1 |
0 |
|
Otitis media |
1 |
0 |
Other reactions in more than 1% of patients but equally or more frequent in the placebo group included pain, tremor, neuralgia, back pain, dyspepsia, dyspnea, and flu syndrome.
There were no clinically important differences between men and women in the types and incidence of adverse reactions. Because there were few patients whose race was reported as other than white, there are insufficient data to support a statement regarding the distribution of adverse reactions by race.
Epilepsy with Partial Onset Seizures (Adjunctive Therapy)
The most common adverse reactions with gabapentin in combination with other antiepileptic drugs in patients > 12 years of age, not seen at an equivalent frequency among placebo-treated patients, were somnolence, dizziness, ataxia, fatigue, and nystagmus.
The most common adverse reactions with gabapentin in combination with other antiepileptic drugs in pediatric patients 3 to 12 years of age, not seen at an equal frequency among placebo-treated patients, were viral infection, fever, nausea and/or vomiting, somnolence, and hostility [see Warnings and Precautions (5.9)].
Approximately 7% of the 2,074 patients > 12 years of age and approximately 7% of the 449 pediatric patients 3 to 12 years of age who received gabapentin in premarketing clinical trials discontinued treatment because of an adverse reaction. The adverse reactions most commonly associated with withdrawal in patients > 12 years of age were somnolence (1.2%), ataxia (0.8%), fatigue (0.6%), nausea and/or vomiting (0.6%), and dizziness (0.6%). The adverse reactions most commonly associated with withdrawal in pediatric patients were emotional lability (1.6%), hostility (1.3%), and hyperkinesia (1.1%).
Table 4 lists adverse reactions that occurred in at least 1% of gabapentin- treated patients >12 years of age with epilepsy participating in placebo- controlled trials and were numerically more common in the gabapentin group. In these studies, either gabapentin or placebo was added to the patient's current antiepileptic drug therapy.
Table 4 Adverse Reactions in Pooled Placebo-Controlled Add-On Trials in Epilepsy Patients >12 Years of Age|
a Plus background antiepileptic drug therapy | ||
|
b Amblyopia was often described as blurred vision. | ||
|
** Gabapentin****a** |
** Placebo****a** | |
|
** Body as a Whole** | ||
|
Fatigue |
11 |
5 |
|
Increased weight |
3 |
2 |
|
Back pain |
2 |
1 |
|
Peripheral edema |
2 |
1 |
|
** Cardiovascular** | ||
|
Vasodilatation |
1 |
0 |
|
** Digestive System** | ||
|
Dyspepsia |
2 |
1 |
|
Dry mouth or throat |
2 |
1 |
|
Constipation |
2 |
1 |
|
Dental abnormalities |
2 |
0 |
|
** Nervous System** | ||
|
Somnolence |
19 |
9 |
|
Dizziness |
17 |
7 |
|
Ataxia |
13 |
6 |
|
Nystagmus |
8 |
4 |
|
Tremor |
7 |
3 |
|
Dysarthria |
2 |
1 |
|
Amnesia |
2 |
0 |
|
Depression |
2 |
1 |
|
Abnormal thinking |
2 |
1 |
|
Abnormal coordination |
1 |
0 |
|
** Respiratory System** | ||
|
Pharyngitis |
3 |
2 |
|
Coughing |
2 |
1 |
|
** Skin and Appendages** | ||
|
Abrasion |
1 |
0 |
|
** Urogenital System** | ||
|
Impotence |
2 |
1 |
|
** Special Senses** | ||
|
Diplopia |
6 |
2 |
|
Amblyopiab |
4 |
1 |
Among the adverse reactions occurring at an incidence of at least 10% in gabapentin-treated patients, somnolence and ataxia appeared to exhibit a positive dose-response relationship.
The overall incidence of adverse reactions and the types of adverse reactions seen were similar among men and women treated with gabapentin. The incidence of adverse reactions increased slightly with increasing age in patients treated with either gabapentin or placebo. Because only 3% of patients (28/921) in placebo-controlled studies were identified as nonwhite (black or other), there are insufficient data to support a statement regarding the distribution of adverse reactions by race.
Table 5 lists adverse reactions that occurred in at least 2% of gabapentin- treated patients, age 3 to 12 years of age with epilepsy participating in placebo-controlled trials, and which were numerically more common in the gabapentin group.
Table 5 Adverse Reactions in a Placebo-Controlled Add-On Trial in Pediatric Epilepsy Patients Age 3 Years to 12 Years|
a Plus background antiepileptic drug therapy | ||
|
** Gabapentin****a** |
** Placebo****a** | |
|
** Body as a Whole** | ||
|
Viral infection |
11 |
3 |
|
Fever |
10 |
3 |
|
Increased weight |
3 |
1 |
|
Fatigue |
3 |
2 |
|
** Digestive System** | ||
|
Nausea and/or vomiting |
8 |
7 |
|
** Nervous System** | ||
|
Somnolence |
8 |
5 |
|
Hostility |
8 |
2 |
|
Emotional lability |
4 |
2 |
|
Dizziness |
3 |
2 |
|
Hyperkinesia |
3 |
1 |
|
** Respiratory System** | ||
|
Bronchitis |
3 |
1 |
|
Respiratory infection |
3 |
1 |
Other reactions in more than 2% of pediatric patients 3 to 12 years of age but equally or more frequent in the placebo group included: pharyngitis, upper respiratory infection, headache, rhinitis, convulsions, diarrhea, anorexia, coughing, and otitis media.
6.2 Postmarketing Experience
The following adverse reactions have been identified during postmarketing use of gabapentin. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Hepatobiliary Disorders: jaundice
Investigations: elevated creatine kinase, elevated liver function tests
Metabolism and Nutrition Disorders: hyponatremia
Musculoskeletal and Connective Tissue Disorder: rhabdomyolysis
Nervous System Disorders: movement disorder
Psychiatric Disorders: agitation
Reproductive System and Breast Disorders: breast enlargement, changes in libido, ejaculation disorders and anorgasmia
Skin and Subcutaneous Tissue Disorders: angioedema [see Warnings and Precautions (5.2)], bullous pemphigoid, erythema multiforme, Stevens-Johnson syndrome.
There are postmarketing reports of life-threatening or fatal respiratory depression in patients taking gabapentin with opioids or other CNS depressants, or in the setting of underlying respiratory impairment [see Warnings and Precautions (5.8)].
There are postmarketing reports of withdrawal symptoms after discontinuation of gabapentin. Reported adverse reactions include, but are not limited to, seizures, depression, suicidal ideation and behavior, agitation, confusion, disorientation, psychotic symptoms, anxiety, insomnia, nausea, pain, sweating, tremor, headache, dizziness, and malaise [see Warnings and Precautions (5.6)].
Most common adverse reactions (incidence ≥ 8% and at least twice that for placebo) were:
- Postherpetic neuralgia: Dizziness, somnolence, and peripheral edema (6.1)
- Epilepsy in patients > 12 years of age: Somnolence, dizziness, ataxia, fatigue, and nystagmus (6.1)
- Epilepsy in patients 3 to 12 years of age: Viral infection, fever, nausea and/or vomiting, somnolence, and hostility (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Zydus Pharmaceuticals (USA) Inc. at 1-877-993-8779 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
7.1 Opioids
Respiratory depression and sedation, sometimes resulting in death, have been reported following coadministration of gabapentin with opioids (e.g., morphine, hydrocodone, oxycodone, buprenorphine) [see Warnings and Precautions (5.8)].
Hydrocodone
Coadministration of gabapentin with hydrocodone decreases hydrocodone exposure [see Clinical Pharmacology (12.3)]. The potential for alteration in hydrocodone exposure and effect should be considered when gabapentin is started or discontinued in a patient taking hydrocodone.
Morphine
When gabapentin is administered with morphine, patients should be observed for signs of CNS depression, such as somnolence, sedation and respiratory depression [see Clinical Pharmacology (12.3)].
7.2 Other Antiepileptic Drugs
Gabapentin is not appreciably metabolized nor does it interfere with the metabolism of commonly coadministered antiepileptic drugs [see Clinical Pharmacology (12.3)].
7.3 Maalox® (aluminum hydroxide, magnesium hydroxide)
The mean bioavailability of gabapentin was reduced by about 20% with concomitant use of an antacid (Maalox®) containing magnesium and aluminum hydroxides. It is recommended that gabapentin be taken at least 2 hours following Maalox administration [see Clinical Pharmacology (12.3)].
7.4 Drug/Laboratory Test Interactions
Because false positive readings were reported with the Ames N-Multistix SG®dipstick test for urinary protein when gabapentin was added to other antiepileptic drugs, the more specific sulfosalicylic acid precipitation procedure is recommended to determine the presence of urine protein.
Concentrations increased by morphine; may need dose adjustment (5.4, 7.1)
DESCRIPTION SECTION
11 DESCRIPTION
The active ingredient in gabapentin tablets, USP is gabapentin USP which has the chemical name 1-(aminomethyl)cyclohexaneacetic acid.
The molecular formula of gabapentin is C9H17NO2 and the molecular weight is 171.24. The structural formula of gabapentin is:
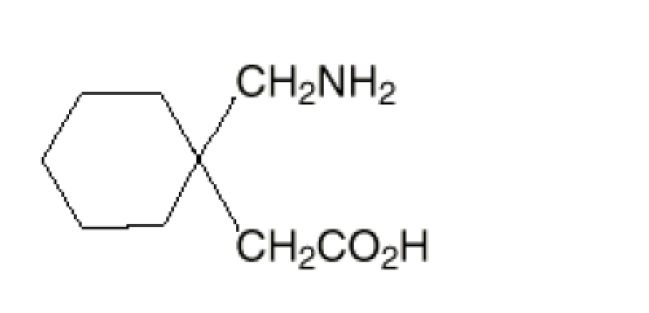
Gabapentin, USP is a white to off-white powder with a pKa1 of 3.7 and a pKa2 of 10.7. It is freely soluble in water, 0.1 N hydrochloric acid, 0.1 N sodium hydroxide and glacial acetic acid; slightly soluble in methanol, very slightly soluble in ethanol, 2-propanol; insoluble in toluene. The log of the partition coefficient (n-octanol/0.05M phosphate buffer) at pH 7.4 is –1.25.
Each gabapentin tablet, USP intended for oral administration contains 600 mg and 800 mg of gabapentin. In addition each tablet contains following inactive ingredients: copovidone, low substituted hydroxypropyl cellulose, magnesium stearate, mannitol, poloxamer, povidone and talc.
SPL UNCLASSIFIED SECTION
Manufactured by:
Zydus Lifesciences Ltd.
Ahmedabad, India
Distributed by:
Zydus Pharmaceuticals (USA) Inc.
Pennington, NJ 08534
Rev.: 09/25
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
2.1 Dosage for Postherpetic Neuralgia
In adults with postherpetic neuralgia, gabapentin may be initiated on Day 1 as a single 300 mg dose, on Day 2 as 600 mg/day (300 mg two times a day), and on Day 3 as 900 mg/day (300 mg three times a day). The dose can subsequently be titrated up as needed for pain relief to a dose of 1,800 mg/day (600 mg three times a day). In clinical studies, efficacy was demonstrated over a range of doses from 1,800 mg/day to 3,600 mg/day with comparable effects across the dose range; however, in these clinical studies, the additional benefit of using doses greater than 1,800 mg/day was not demonstrated.
2.2 Dosage for Epilepsy with Partial Onset Seizures
Patients 12 Years of Age and Above
The starting dose is 300 mg three times a day. The recommended maintenance dose of gabapentin is 300 mg to 600 mg three times a day. Dosages up to 2,400 mg/day have been administered in long-term clinical studies. Doses of 3,600 mg/day have also been administered to a small number of patients for a relatively short duration. Administer gabapentin three times a day using 600 mg or 800 mg tablets. The maximum time between doses should not exceed 12 hours.
Pediatric Patients Age 3****Years to 11 Years
The starting dose range is 10 mg/kg/day to 15 mg/kg/day, given in three divided doses, and the recommended maintenance dose reached by upward titration over a period of approximately 3 days. The recommended maintenance dose of gabapentin in patients 3 years to 4 years of age is 40 mg/kg/day, given in three divided doses. The recommended maintenance dose of gabapentin in patients 5 years to 11 years of age is 25 mg/kg/day to 35 mg/kg/day, given in three divided doses. Dosages up to 50 mg/kg/day have been administered in a long-term clinical study. The maximum time interval between doses should not exceed 12 hours.
2.3 Dosage Adjustment in Patients with Renal Impairment
Dosage adjustment in patients 12 years of age and older with renal impairment or undergoing hemodialysis is recommended, as follows (see dosing recommendations above for effective doses in each indication):
Table 1 Gabapentin Dosage Based on Renal Function|
TID = Three times a day; BID = Two times a day; QD = Single daily dose | ||||||
|
a For patients with creatinine clearance < 15 mL/min, reduce daily dose in proportion to creatinine clearance (e.g., patients with a creatinine clearance of 7.5 mL/min should receive one-half the daily dose that patients with a creatinine clearance of 15 mL/min receive). | ||||||
|
b Patients on hemodialysis should receive maintenance doses based on estimates of creatinine clearance as indicated in the upper portion of the table and a supplemental post-hemodialysis dose administered after each 4 hours of hemodialysis as indicated in the lower portion of the table. | ||||||
|
** Renal Function Creatinine Clearance (mL/min)** |
** Total Daily Dose Range** |
** Dose Regimen** | ||||
|
≥ 60 |
900 to 3,600 |
300 TID |
400 TID |
600 TID |
800 TID |
1,200 TID |
|
400 to 1,400 |
200 BID |
300 BID |
400 BID |
500 BID |
700 BID |
|
200 to 700 |
200 QD |
300 QD |
400 QD |
500 QD |
700 QD |
|
15a |
100 to 300 |
100 QD |
125 QD |
150 QD |
200 QD |
300 QD |
|
Post-Hemodialysis Supplemental Dose (mg)b | ||||||
|
Hemodialysis |
125b |
150b |
200b |
250b |
350b |
Creatinine clearance (CLCr) is difficult to measure in outpatients. In patients with stable renal function, creatinine clearance can be reasonably well estimated using the equation of Cockcroft and Gault:
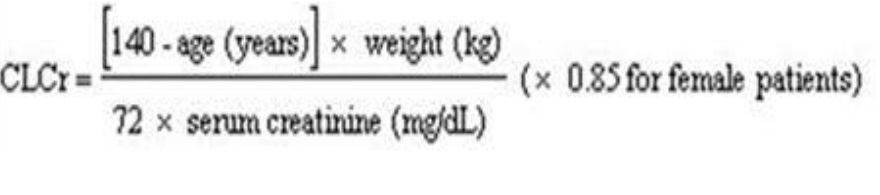
The use of gabapentin in patients less than 12 years of age with compromised renal function has not been studied.
2.4 Dosage in Elderly
Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and dose should be adjusted based on creatinine clearance values in these patients.
2.5 Administration Information
Administer gabapentin tablets orally with or without food.
Inform patients that, should they divide the scored 600 mg or 800 mg gabapentin tablets in order to administer a half-tablet, they should take the unused half-tablet as the next dose. Half-tablets not used within 28 days of dividing the scored tablet should be discarded.
If gabapentin dose is reduced, discontinued, or substituted with an alternative medication, this should be done gradually over a minimum of 1 week (a longer period may be needed at the discretion of the prescriber).
- Postherpetic Neuralgia (2.1)
- Dose can be titrated up as needed to a dose of 1800 mg/day
- Day 1: Single 300 mg dose
- Day 2: 600 mg/day (i.e., 300 mg two times a day)
- Day 3: 900 mg/day (i.e., 300 mg three times a day)
- Epilepsy with Partial Onset Seizures (2.2)
- Patients 12 years of age and older: starting dose is 300 mg three times daily; may be titrated up to 600 mg three times daily
- Patients 3 to 11 years of age: starting dose range is 10 to 15 mg/kg/day, given in three divided doses; recommended dose in patients 3 to 4 years of age is 40 mg/kg/day, given in three divided doses; the recommended dose in patients 5 to 11 years of age is 25 to 35 mg/kg/day, given in three divided doses. The recommended dose is reached by upward titration over a period of approximately 3 days
- Dose should be adjusted in patients with reduced renal function (2.3, 2.4)
SPL MEDGUIDE SECTION
|
** Medication Guide** | ||
|
** What is the most important information I should know about**** gabapentin
tablets?** ** Gabapentin tablets can cause serious side effects including:**
** How can I watch for early symptoms of suicidal thoughts and actions?**
Call your healthcare provider between visits as needed, especially if you are
worried about symptoms.
** 2**** Changes in behavior and thinking.** Using gabapentin tablets in
children 3 to 12 years of age can cause emotional changes, aggressive
behavior, problems with concentration, changes in school performance,
restlessness, and hyperactivity.
These symptoms may be the first signs of a serious reaction. A healthcare
provider should examine you to decide if you should continue taking gabapentin
tablets. | ||
|
|
|
|
|
|
|
Be sure that your caregiver or family members know which symptoms may be
serious so they can call your healthcare provider or get medical help if you
are unable to seek treatment on your own. | ||
|
** What are gabapentin tablets?**
It is not known if gabapentin tablets are safe and effective to treat:
| ||
|
** Do not take**** gabapentin tablets if you:**
| ||
|
** Before taking**** gabapentin tablets**** , tell your healthcare provider about all of your medical conditions including if you:**
*** Pregnancy Registry:** If you become pregnant while taking gabapentin tablets, talk to your healthcare provider about registering with the North American Antiepileptic Drug (NAAED) Pregnancy Registry. The purpose of this registry is to collect information about the safety of antiepileptic drugs during pregnancy. You can enroll in this registry by calling 1-888-233-2334 or visiting http://www.aedpregnancyregistry.org/.
** Tell your healthcare provider about all the medicines you take** ,
including prescription and over-the-counter medicines, vitamins, and herbal
supplements.
Taking gabapentin tablets with certain other medicines can cause side effects
or affect how well they work.** Do not** start or stop other medicines
without talking to your healthcare provider. | ||
|
** How should I take**** gabapentin tablets?**
*** Do not** change your dose of gabapentin tablets without talking to your healthcare provider. *** Do not** stop taking gabapentin tablets without talking to your healthcare provider first. If you stop taking gabapentin tablets suddenly, you may develop side effects.
| ||
|
** What should I avoid while taking**** gabapentin tablets?** *** Do not** drink alcohol or take other medicines that make you sleepy or dizzy while taking gabapentin tablets without first talking with your healthcare provider. Taking gabapentin tablets with alcohol or drugs that cause sleepiness or dizziness may make your sleepiness or dizziness worse. *** Do not** drive, operate heavy machinery, or do other dangerous activities until you know how gabapentin tablets affects you. Gabapentin tablets can slow your thinking and motor skills. | ||
|
** What are the possible side effects of**** gabapentin tablets?**
** The most common side effects of**** gabapentin tablets include:** | ||
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| ||
|
Tell your healthcare provider if you have any side effect that bothers you or
that does not go away. | ||
|
** How should I store**** gabapentin tablets?**
** Keep**** gabapentin tablets and all medicines out of the reach of children.** | ||
|
** General information about the safe and effective use of**** gabapentin
tablets** | ||
|
** What are the ingredients in**** gabapentin tablets?** | ||
|
** Manufactured by:** | ||
|
Rev.: 09/25 |
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
14.1 Postherpetic Neuralgia
Gabapentin was evaluated for the management of postherpetic neuralgia (PHN) in two randomized, double-blind, placebo-controlled, multicenter studies. The intent-to-treat (ITT) population consisted of a total of 563 patients with pain for more than 3 months after healing of the herpes zoster skin rash (Table 6).
Table 6 Controlled PHN Studies: Duration, Dosages, and Number of Patients|
aGiven in 3 divided doses (TID) | ||||
|
Study |
Study Duration |
Gabapentin (mg/day)a Target Dose |
Patients Receiving Gabapentin |
Patients Receiving Placebo |
|
1 |
8 weeks |
3,600 |
113 |
116 |
|
2 |
7 weeks |
1,800, 2,400 |
223 |
111 |
|
Total |
336 |
227 |
Each study included a 7- week or 8-week double-blind phase (3 weeks or 4 weeks of titration and 4 weeks of fixed dose). Patients initiated treatment with titration to a maximum of 900 mg/day gabapentin over 3 days. Dosages were then to be titrated in 600 mg/day to 1,200 mg/day increments at 3-day to 7-day intervals to the target dose over 3 weeks to 4 weeks. Patients recorded their pain in a daily diary using an 11-point numeric pain rating scale ranging from 0 (no pain) to 10 (worst possible pain). A mean pain score during baseline of at least 4 was required for randomization. Analyses were conducted using the ITT population (all randomized patients who received at least one dose of study medication).
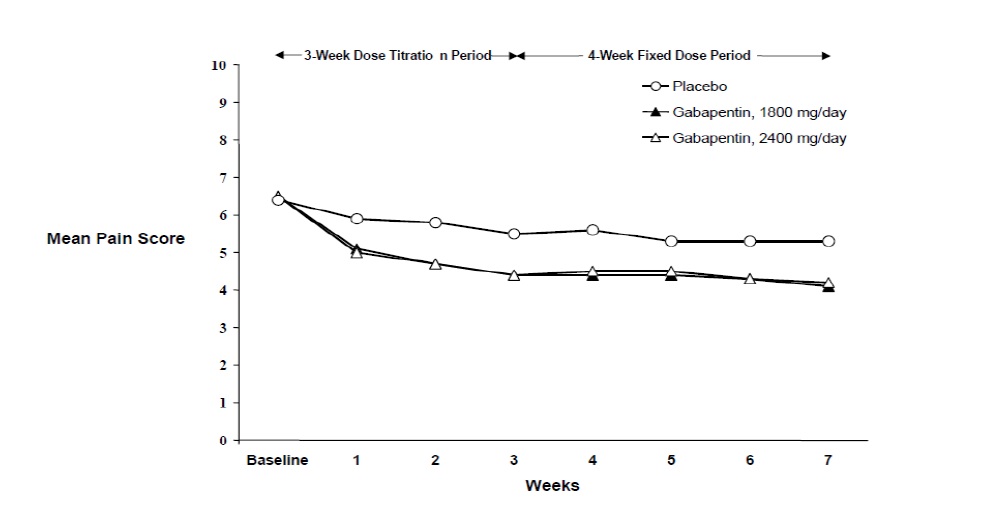
Both studies demonstrated efficacy compared to placebo at all doses tested.
The reduction in weekly mean pain scores was seen by Week 1 in both studies, and were maintained to the end of treatment. Comparable treatment effects were observed in all active treatment arms. Pharmacokinetic/pharmacodynamic modeling provided confirmatory evidence of efficacy across all doses. Figures 1 and 2 show pain intensity scores over time for Studies 1 and 2.
Figure 1
Weekly Mean Pain Scores (Observed Cases in ITT Population): Study 1
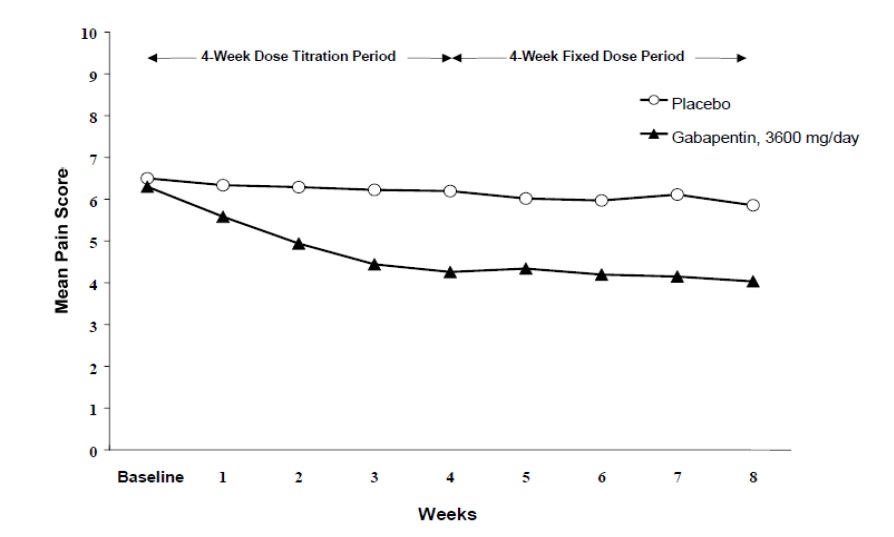
Figure 2
Weekly Mean Pain Scores (Observed Cases in ITT Population): Study 2
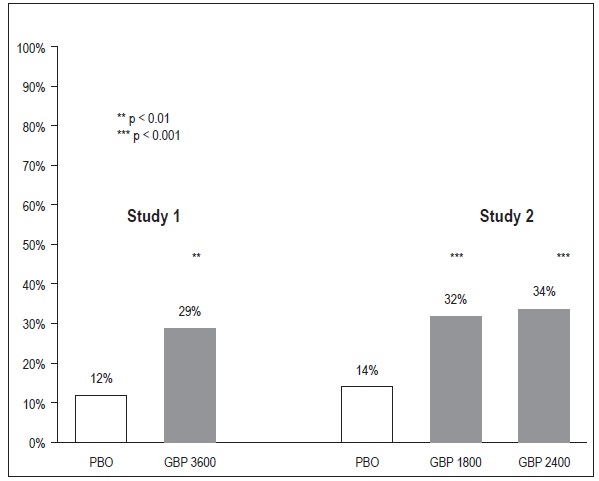
The proportion of responders (those patients reporting at least 50% improvement in endpoint pain score compared to baseline) was calculated for each study (Figure 3).
Figure 3
Proportion of Responders (patients with ≥ 50% reduction in pain score) at Endpoint: Controlled PHN Studies
14.2 Epilepsy for Partial Onset Seizures (Adjunctive Therapy)
The effectiveness of gabapentin as adjunctive therapy (added to other antiepileptic drugs) was established in multicenter placebo-controlled, double-blind, parallel-group clinical trials in adult and pediatric patients (3 years and older) with refractory partial seizures.
Evidence of effectiveness was obtained in three trials conducted in 705 patients (age 12 years and above) and one trial conducted in 247 pediatric patients (3 to 12 years of age). The patients enrolled had a history of at least 4 partial seizures per month in spite of receiving one or more antiepileptic drugs at therapeutic levels and were observed on their established antiepileptic drug regimen during a 12-week baseline period (6 weeks in the study of pediatric patients). In patients continuing to have at least 2 (or 4 in some studies) seizures per month, gabapentin or placebo was then added on to the existing therapy during a 12-week treatment period. Effectiveness was assessed primarily on the basis of the percent of patients with a 50% or greater reduction in seizure frequency from baseline to treatment (the "responder rate") and a derived measure called response ratio, a measure of change defined as (T -B)/(T + B), in which B is the patient's baseline seizure frequency and T is the patient's seizure frequency during treatment. Response ratio is distributed within the range -1 to +1. A zero value indicates no change while complete elimination of seizures would give a value of -1; increased seizure rates would give positive values. A response ratio of -0.33 corresponds to a 50% reduction in seizure frequency. The results given below are for all partial seizures in the intent-to-treat (all patients who received any doses of treatment) population in each study, unless otherwise indicated.
One study compared gabapentin 1,200 mg/day, in three divided doses with placebo. Responder rate was 23% (14/61) in the gabapentin group and 9% (6/66) in the placebo group; the difference between groups was statistically significant. Response ratio was also better in the gabapentin group (-0.199) than in the placebo group (-0.044), a difference that also achieved statistical significance.
A second study compared primarily gabapentin 1,200 mg/day, in three divided doses (N=101), with placebo (N=98). Additional smaller gabapentin dosage groups (600 mg/day, N=53; 1,800 mg/day, N=54) were also studied for information regarding dose response. Responder rate was higher in the gabapentin 1,200 mg/day group (16%) than in the placebo group (8%), but the difference was not statistically significant. The responder rate at 600 mg (17%) was also not significantly higher than in the placebo, but the responder rate in the 1,800 mg group (26%) was statistically significantly superior to the placebo rate. Response ratio was better in the gabapentin 1,200 mg/day group (-0.103) than in the placebo group (-0.022); but this difference was also not statistically significant (p = 0.224). A better response was seen in the gabapentin 600 mg/day group (-0.105) and 1,800 mg/day group (-0.222) than in the 1,200 mg/day group, with the 1,800 mg/day group achieving statistical significance compared to the placebo group.
A third study compared gabapentin 900 mg/day, in three divided doses (N=111), and placebo (N=109). An additional gabapentin 1,200 mg/day dosage group (N=52) provided dose-response data. A statistically significant difference in responder rate was seen in the gabapentin 900 mg/day group (22%) compared to that in the placebo group (10%). Response ratio was also statistically significantly superior in the gabapentin 900 mg/day group (-0.119) compared to that in the placebo group (-0.027), as was response ratio in 1,200 mg/day gabapentin (-0.184) compared to placebo.
Analyses were also performed in each study to examine the effect of gabapentin on preventing secondarily generalized tonic-clonic seizures. Patients who experienced a secondarily generalized tonic-clonic seizure in either the baseline or in the treatment period in all three placebo-controlled studies were included in these analyses. There were several response ratio comparisons that showed a statistically significant advantage for gabapentin compared to placebo and favorable trends for almost all comparisons.
Analysis of responder rate using combined data from all three studies and all doses (N=162, gabapentin; N=89, placebo) also showed a significant advantage for gabapentin over placebo in reducing the frequency of secondarily generalized tonic-clonic seizures.
In two of the three controlled studies, more than one dose of gabapentin was used. Within each study, the results did not show a consistently increased response to dose. However, looking across studies, a trend toward increasing efficacy with increasing dose is evident (see Figure 4).
Figure 4
Responder Rate in Patients Receiving gabapentin Expressed as a Difference from Placebo by Dose and Study: Adjunctive Therapy Studies in Patients ≥ 12 Years of Age with Partial Seizures
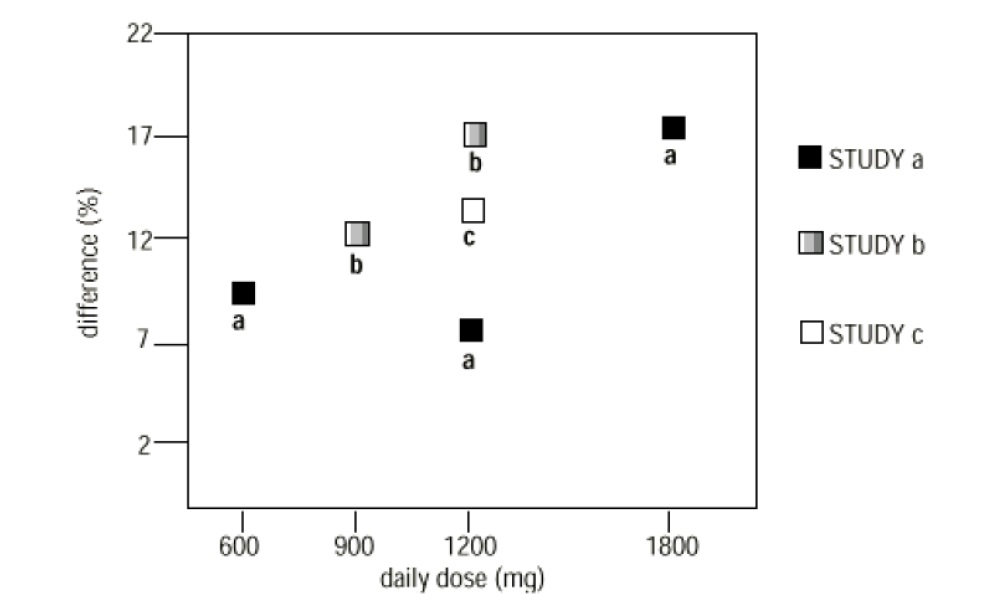
In the figure, treatment effect magnitude, measured on the Y axis in terms of the difference in the proportion of gabapentin and placebo-assigned patients attaining a 50% or greater reduction in seizure frequency from baseline, is plotted against the daily dose of gabapentin administered (X axis).
Although no formal analysis by gender has been performed, estimates of response (Response Ratio) derived from clinical trials (398 men, 307 women) indicate no important gender differences exist. There was no consistent pattern indicating that age had any effect on the response to gabapentin. There were insufficient numbers of patients of races other than Caucasian to permit a comparison of efficacy among racial groups.
A fourth study in pediatric patients age 3 years to 12 years compared 25 mg/kg/day to 35 mg/kg/day gabapentin (N=118) with placebo (N=127). For all partial seizures in the intent-to-treat population, the response ratio was statistically significantly better for the gabapentin group (-0.146) than for the placebo group (-0.079). For the same population, the responder rate for gabapentin (21%) was not significantly different from placebo (18%).
A study in pediatric patients age 1 month to 3 years compared 40 mg/kg/day gabapentin (N=38) with placebo (N=38) in patients who were receiving at least one marketed antiepileptic drug and had at least one partial seizure during the screening period (within 2 weeks prior to baseline). Patients had up to 48 hours of baseline and up to 72 hours of double-blind video EEG monitoring to record and count the occurrence of seizures. There were no statistically significant differences between treatments in either the response ratio or responder rate.
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Administration Information
Inform patients that gabapentin is taken orally with or without food. Inform patients that, should they divide the scored 600 mg or 800 mg tablet in order to administer a half-tablet, they should take the unused half-tablet as the next dose. Advise patients to discard half-tablets not used within 28 days of dividing the scored tablet.
Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)/Multiorgan Hypersensitivity
Prior to initiation of treatment with gabapentin, instruct patients that a rash or other signs or symptoms of hypersensitivity (such as fever or lymphadenopathy) may herald a serious medical event and that the patient should report any such occurrence to a healthcare provider immediately [see Warnings and Precautions (5.1)].
Anaphylaxis and Angioedema
Advise patients to discontinue gabapentin and seek medical care if they develop signs or symptoms of anaphylaxis or angioedema [see Warnings and Precautions (5.2)].
Dizziness and Somnolence and Effects on Driving and Operating Heavy Machinery
Advise patients that gabapentin may cause dizziness, somnolence, and other symptoms and signs of CNS depression. Other drugs with sedative properties may increase these symptoms. Accordingly, although patients' ability to determine their level of impairment can be unreliable, advise them neither to drive a car nor to operate other complex machinery until they have gained sufficient experience on gabapentin to gauge whether or not it affects their mental and/or motor performance adversely. Inform patients that it is not known how long this effect lasts [see Warnings and Precautions (5.3) and Warnings and Precautions (5.4)].
Suicidal Thinking and Behavior
Counsel the patient, their caregivers, and families that AEDs, including gabapentin, may increase the risk of suicidal thoughts and behavior. Advise patients of the need to be alert for the emergence or worsening of symptoms of depression, any unusual changes in mood or behavior, or the emergence of suicidal thoughts, behavior, or thoughts about self-harm. Instruct patients to report behaviors of concern immediately to healthcare providers [see Warnings and Precautions (5.5)]. Also, inform patients who plan to or have discontinued gabapentin that suicidal thoughts and behavior can appear even after the drug is stopped.
Respiratory Depression
Inform patients about the risk of respiratory depression. Include information that the risk is greatest for those using concomitant CNS depressants (such as opioid analgesics) or those with underlying respiratory impairment. Teach patients how to recognize respiratory depression and advise them to seek medical attention immediately if it occurs [see Warnings and Precautions (5.8)].
Use in Pregnancy
Instruct patients to notify their healthcare provider if they are pregnant or intend to become pregnant during therapy, and to notify their healthcare provider if they are breast feeding or intend to breast feed during therapy [see Use in Specific Populations (8.1) and (8.2)].
Encourage patients to enroll in the NAAED Pregnancy Registry if they become pregnant. This registry is collecting information about the safety of antiepileptic drugs during pregnancy. To enroll, patients can call the toll free number 1-888-233-2334 [see Use in Specific Populations (8.1)].
