Recuren Plus Arnica Bruise Cream
3dbe6e5b-1ca7-6941-e063-6294a90ab042
HUMAN OTC DRUG LABEL
Sep 1, 2025
REGENEREN PLUS LTD
DUNS: 226204743
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Arnica Montana 7%
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (11)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
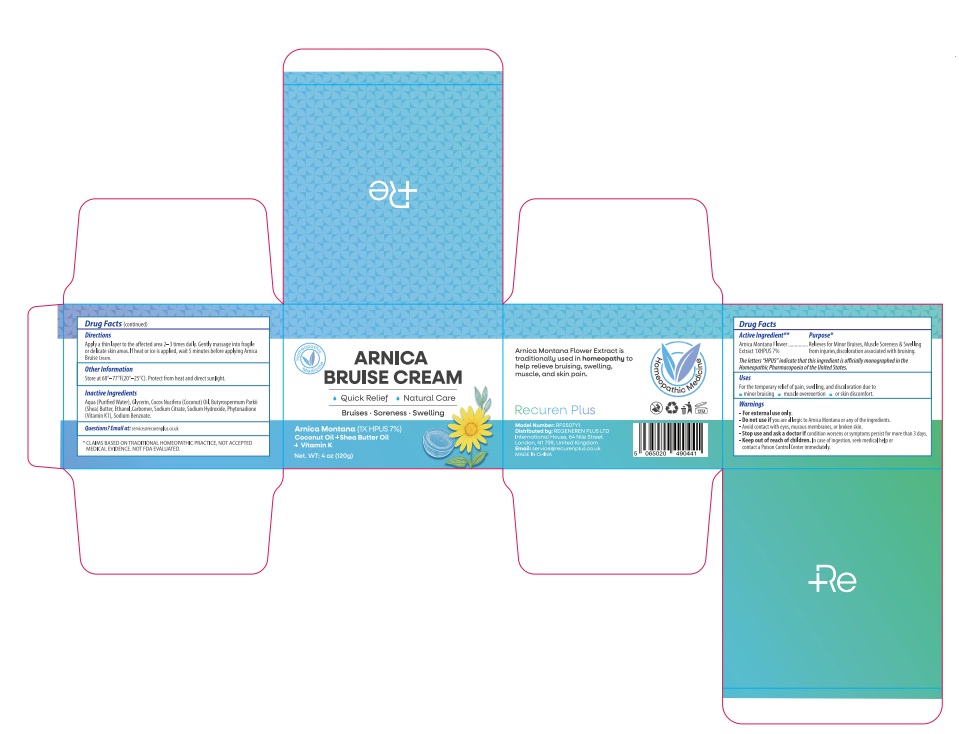
INDICATIONS & USAGE SECTION
For the temporary relief of pain, swelling, and discoloration due to minor bruising, muscle overexertion, or skin discomfort.
OTC - ACTIVE INGREDIENT SECTION
Arnica montana 1X HPUS 7%
The letters "HPUS" indicate that the components in this product are officially monographed in the Homeopathic Pharmacopoeia of the United States.
OTC - PURPOSE SECTION
Relieves for Minor Bruises, Muscle Soreness & Swelling from injuries,discoloration associated with bruising.
WARNINGS SECTION
For external use only.
if you are allergic to Arnica Montana or any of the ingredients.
Avoid contact with eyes, mucous membranes, or broken skin.
Stop use and ask a doctor if condition worsens or symptoms persist for more than 3 days.
Keep out of reach of children. In case of ingestion, seek medical help or contact a Poison Control Center immediately.
DOSAGE & ADMINISTRATION SECTION
Apply a thin layer to the affected area 2–3 times daily. Gently massage into fragile or delicate skin areas. If heat or ice is applied, wait 5 minutes before applying Arnica Bruise Cream.
STORAGE AND HANDLING SECTION
Store at 68°–77°F(20°–25°C). Protect from heat and direct sunlight.
INACTIVE INGREDIENT SECTION
Aqua (Purified Water), Glycerin, Cocos Nucifera (Coconut) Oil, Butyrospermum Parkii (Shea) Butter, Ethanol,Carbomer, Sodium Citrate, Sodium Hydroxide, Phytonadione (Vitamin K1), Sodium Benzoate.
OTC - QUESTIONS SECTION
Email at: service@recurenplus.co.uk
