Everolimus
These highlights do not include all the information needed to use safely and effectively. See full prescribing information for . for oral use Initial U.S. Approval: 2009
e3bca36b-29ed-4a2b-a1be-a57b6af805f6
HUMAN PRESCRIPTION DRUG LABEL
Aug 25, 2025
Teva Pharmaceuticals USA, Inc.
DUNS: 001627975
Products 4
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Everolimus
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
Everolimus
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
Everolimus
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
Everolimus
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC 0093-7769-24
Everolimus Tablets
10 mg
Each tablet contains 10 mg everolimus, USP
For more information, call 1-888-838-2872.
Carton contains 4 individual blister cards of 7 tablets.
Rx only
28 Tablets

INDICATIONS & USAGE SECTION
1 INDICATIONS AND USAGE
1.1 Hormone Receptor-Positive, HER2-Negative Breast Cancer
Everolimus tablets are indicated for the treatment of postmenopausal women
with advanced hormone
receptor-positive, HER2-negative breast cancer in combination with exemestane,
after failure of treatment with letrozole or anastrozole.
1.2 Neuroendocrine Tumors (NET)
Everolimus tablets are indicated for the treatment of adult patients with progressive neuroendocrine tumors of pancreatic origin (PNET) with unresectable, locally advanced or metastatic disease.
Everolimus tablets are indicated for the treatment of adult patients with
progressive, well-differentiated,
non-functional NET of gastrointestinal (GI) or lung origin with unresectable,
locally advanced or metastatic disease.
Limitations of Use: Everolimus tablets are not indicated for the treatment of patients with functional carcinoid tumors [see Clinical Studies (14.2)].
1.3 Renal Cell Carcinoma (RCC)
Everolimus tablets are indicated for the treatment of adult patients with advanced RCC after failure of treatment with sunitinib or sorafenib.
1.4 Tuberous Sclerosis Complex (TSC)-Associated Renal Angiomyolipoma
Everolimus tablets are indicated for the treatment of adult patients with renal angiomyolipoma and TSC, not requiring immediate surgery.
1.5 Tuberous Sclerosis Complex (TSC)-Associated Subependymal Giant Cell
Astrocytoma (SEGA)
Everolimus tablets are indicated in adult and pediatric patients aged 1 year and older with TSC for the treatment of SEGA that requires therapeutic intervention but cannot be curatively resected.
Everolimus tablets are a kinase inhibitor indicated for the treatment of:
- Postmenopausal women with advanced hormone receptor-positive, HER2-negative breast cancer in combination with exemestane after failure of treatment with letrozole or anastrozole. (1.1)
- Adults with progressive neuroendocrine tumors of pancreatic origin (PNET) and adults with progressive, well-differentiated, non-functional neuroendocrine tumors (NET) of gastrointestinal (GI) or lung origin that are unresectable, locally advanced or metastatic.
Limitations of Use: Everolimus tablets are not indicated for the treatment of patients with functional carcinoid tumors. (1.2)
- Adults with advanced renal cell carcinoma (RCC) after failure of treatment with sunitinib or sorafenib. (1.3)
- Adults with renal angiomyolipoma and tuberous sclerosis complex (TSC), not requiring immediate surgery. (1.4)
Everolimus tablets are a kinase inhibitor indicated for the treatment of adult and pediatric patients aged 1 year and older with TSC who have subependymal giant cell astrocytoma (SEGA) that requires therapeutic intervention but cannot be curatively resected. (1.5)
CONTRAINDICATIONS SECTION
4 CONTRAINDICATIONS
Everolimus is contraindicated in patients with clinically significant hypersensitivity to everolimus or to other rapamycin derivatives [see Warnings and Precautions (5.3)].
Clinically significant hypersensitivity to everolimus or to other rapamycin derivatives. (4)
WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Non-infectious Pneumonitis
Non-infectious pneumonitis is a class effect of rapamycin derivatives. Non- infectious pneumonitis was reported in up to 19% of patients treated with everolimus in clinical trials, some cases were reported with pulmonary hypertension (including pulmonary arterial hypertension) as a secondary event. The incidence of Grade 3 and 4 non-infectious pneumonitis was up to 4% and up to 0.2%, respectively [see Adverse Reactions (6.1)]. Fatal outcomes have been observed.
Consider a diagnosis of non-infectious pneumonitis in patients presenting with non-specific respiratory signs and symptoms. Consider opportunistic infections, such as pneumocystis jiroveci pneumonia (PJP) in the differential diagnosis. Advise patients to report promptly any new or worsening respiratory symptoms.
Continue everolimus without dose alteration in patients who develop
radiological changes suggestive of
non-infectious pneumonitis and have few or no symptoms. Imaging appears to
overestimate the incidence of clinical pneumonitis.
For Grade 2 to 4 non-infectious pneumonitis, withhold or permanently discontinue everolimus based on severity [see Dosage and Administration (2.9)]. Corticosteroids may be indicated until clinical symptoms resolve. Administer prophylaxis for PJP when concomitant use of corticosteroids or other immunosuppressive agents are required. The development of pneumonitis has been reported even at a reduced dose.
5.2 Infections
Everolimus has immunosuppressive properties and may predispose patients to bacterial, fungal, viral, or protozoal infections, including infections with opportunistic pathogens [see Adverse Reactions (6.1)]. Localized and systemic infections, including pneumonia, mycobacterial infections, other bacterial infections, invasive fungal infections (e.g., aspergillosis, candidiasis, or PJP), and viral infections (e.g., reactivation of hepatitis B virus) have occurred. Some of these infections have been severe (e.g., sepsis, septic shock, or resulting in multisystem organ failure) or fatal. The incidence of Grade 3 and 4 infections was up to 10% and up to 3%, respectively. The incidence of serious infections was reported at a higher frequency in patients <6 years of age [see Use in Specific Populations (8.4)].
Complete treatment of preexisting invasive fungal infections prior to starting treatment. Monitor for signs and symptoms of infection. Withhold or permanently discontinue everolimus based on severity of infection [see Dosage and Administration (2.9)].
Administer prophylaxis for PJP when concomitant use of corticosteroids or other immunosuppressive agents are required.
5.3 Severe Hypersensitivity Reactions
Hypersensitivity reactions to everolimus have been observed and include anaphylaxis, dyspnea, flushing, chest pain, and angioedema (e.g., swelling of the airways or tongue, with or without respiratory impairment) [see Contraindications (4)]. The incidence of Grade 3 hypersensitivity reactions was up to 1%. Permanently discontinue everolimus for the development of clinically significant hypersensitivity.
5.4 Angioedema with Concomitant Use of Angiotensin-Converting Enzyme (ACE)
Inhibitors
Patients taking concomitant ACE inhibitors with everolimus may be at increased risk for angioedema (e.g., swelling of the airways or tongue, with or without respiratory impairment). In a pooled analysis of randomized double-blind oncology clinical trials, the incidence of angioedema in patients taking everolimus with an ACE inhibitor was 6.8% compared to 1.3% in the control arm with an ACE inhibitor. Permanently discontinue everolimus for angioedema.
5.5 Stomatitis
Stomatitis, including mouth ulcers and oral mucositis, has occurred in patients treated with everolimus at an incidence ranging from 44% to 78% across clinical trials. Grades 3 to 4 stomatitis was reported in 4% to 9% of patients [see Adverse Reactions (6.1)]. Stomatitis most often occurs within the first 8 weeks of treatment. When starting everolimus, initiating dexamethasone alcohol-free oral solution as a swish and spit mouthwash reduces the incidence and severity of stomatitis [see Adverse Reactions (6.1)]. If stomatitis does occur, mouthwashes and/or other topical treatments are recommended. Avoid alcohol-, hydrogen peroxide-, iodine-, or thyme-containing products, as they may exacerbate the condition. Do not administer antifungal agents, unless fungal infection has been diagnosed.
5.6 Renal Failure
Cases of renal failure (including acute renal failure), some with a fatal outcome, have occurred in patients taking everolimus. Elevations of serum creatinine and proteinuria have been reported in patients taking everolimus [see Adverse Reactions (6.1)]. The incidence of Grade 3 and 4 elevations of serum creatinine was up to 2% and up to 1%, respectively. The incidence of Grade 3 and 4 proteinuria was up to 1% and up to 0.5%, respectively. Monitor renal function prior to starting everolimus and annually thereafter. Monitor renal function at least every 6 months in patients who have additional risk factors for renal failure.
5.7 Risk of Impaired Wound Healing
Impaired wound healing can occur in patients who receive drugs that inhibit the VEGF signaling pathway. Therefore, everolimus has the potential to adversely affect wound healing.
Withhold everolimus for at least 1 week prior to elective surgery. Do not administer for at least 2 weeks following major surgery and until adequate wound healing. The safety of resumption of treatment upon resolution of wound healing complications has not been established.
5.8 Geriatric Patients
In the randomized hormone receptor-positive, HER2-negative breast cancer study (BOLERO-2), the incidence of deaths due to any cause within 28 days of the last everolimus dose was 6% in patients ≥65 years of age compared to 2% in patients <65 years of age. Adverse reactions leading to permanent treatment discontinuation occurred in 33% of patients ≥65 years of age compared to 17% in patients <65 years of age. Careful monitoring and appropriate dose adjustments for adverse reactions are recommended [see Dosage and Administration (2.9), Use in Specific Populations (8.5)].
5.9 Metabolic Disorders
Hyperglycemia, hypercholesterolemia, and hypertriglyceridemia have been
reported in patients taking everolimus at an incidence up to 75%, 86%, and
73%, respectively. The incidence of these Grade 3 and 4 laboratory
abnormalities was up to 15% and up to 0.4%, respectively [see Adverse Reactions (6.1)]. In
non-diabetic patients, monitor fasting serum glucose prior to starting
everolimus and annually thereafter. In diabetic patients, monitor fasting
serum glucose more frequently as clinically indicated. Monitor lipid profile
prior to starting everolimus and annually thereafter. When possible, achieve
optimal glucose and lipid control prior to starting everolimus. For Grade 3 to
4 metabolic events, withhold or permanently discontinue everolimus based on
severity [see Dosage and Administration (2.9)].
5.10 Myelosuppression
Anemia, lymphopenia, neutropenia, and thrombocytopenia have been reported in patients taking everolimus. The incidence of these Grade 3 and 4 laboratory abnormalities was up to 16% and up to 2%, respectively [see Adverse Reactions (6.1)]. Monitor complete blood count (CBC) prior to starting everolimus every 6 months for the first year of treatment and annually thereafter. Withhold or permanently discontinue everolimus based on severity [see Dosage and Administration (2.9)].
5.11 Risk of Infection or Reduced Immune Response with Vaccination
The safety of immunization with live vaccines during everolimus therapy has not been studied. Due to the potential increased risk of infection, avoid the use of live vaccines and close contact with individuals who have received live vaccines during treatment with everolimus. Due to the potential increased risk of infection or reduced immune response with vaccination, complete the recommended childhood series of vaccinations according to American Council on Immunization Practices (ACIP) guidelines prior to the start of therapy. An accelerated vaccination schedule may be appropriate.
5.12 Radiation Sensitization and Radiation Recall
Radiation sensitization and recall, in some cases severe, involving cutaneous and visceral organs (including radiation esophagitis and pneumonitis) have been reported in patients treated with radiation prior to, during, or subsequent to everolimus treatment [see Adverse Reactions (6.2)].
Monitor patients closely when everolimus is administered during or sequentially with radiation treatment.
5.13 Embryo-Fetal Toxicity
Based on animal studies and the mechanism of action, everolimus can cause fetal harm when administered to a pregnant woman. In animal studies, everolimus caused embryo-fetal toxicities in rats when administered during the period of organogenesis at maternal exposures that were lower than human exposures at the clinical dose of 10 mg once daily. Advise pregnant women of the potential risk to a fetus. Advise female patients of reproductive potential to avoid becoming pregnant and to use effective contraception during treatment with everolimus and for 8 weeks after the last dose. Advise male patients with female partners of reproductive potential to use effective contraception during treatment with everolimus and for 4 weeks after the last dose [see Use in Specific Populations (8.1, 8.3)].
- Non-Infectious Pneumonitis: Monitor for clinical symptoms or radiological changes. Withhold or permanently discontinue based on severity. (2.9, 5.1)
- Infections: Monitor for signs and symptoms of infection. Withhold or permanently discontinue based on severity. (2.9, 5.2)
- Severe Hypersensitivity Reactions: Permanently discontinue for clinically significant hypersensitivity. (5.3)
- Angioedema: Patients taking concomitant angiotensin-converting-enzyme (ACE) inhibitors may be at increased risk for angioedema. Permanently discontinue for angioedema. (5.4, 7.2)
- Stomatitis: Initiate dexamethasone alcohol-free mouthwash when starting treatment. (5.5, 6.1)
- Renal Failure: Monitor renal function prior to treatment and periodically thereafter. (5.6)
- Risk of Impaired Wound Healing: Withhold for at least 1 week prior to elective surgery. Do not administer for at least 2 weeks following major surgery and until adequate wound healing. The safety of resumption of treatment after resolution of wound healing complications has not been established. (5.7)
- Geriatric Patients: Monitor and adjust dose for adverse reactions. (5.8)
- Metabolic Disorders: Monitor serum glucose and lipids prior to treatment and periodically thereafter. Withhold or permanently discontinue based on severity. (2.9, 5.9)
- Myelosuppression: Monitor hematologic parameters prior to treatment and periodically thereafter. Withhold or permanently discontinue based on severity. (2.9, 5.10)
- Risk of Infection or Reduced Immune Response with Vaccination: Avoid live vaccines and close contact with those who have received live vaccines. Complete recommended childhood vaccinations prior to starting treatment. (5.11)
- Radiation Sensitization and Radiation Recall: Severe radiation reactions may occur. (5.12, 6.2)
- Embryo-Fetal Toxicity: Can cause fetal harm. Advise patients of reproductive potential of the potential risk to a fetus and to use effective contraception. (5.13, 8.1, 8.3)
ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
The following serious adverse reactions are described elsewhere in the labeling:
- Non-Infectious Pneumonitis [see Warnings and Precautions (5.1)]
- Infections [see Warnings and Precautions (5.2)]
- Severe Hypersensitivity Reactions [see Warnings and Precautions (5.3)]
- Angioedema with Concomitant Use of ACE inhibitors [see Warnings and Precautions (5.4)]
- Stomatitis [see Warnings and Precautions (5.5)]
- Renal Failure [see Warnings and Precautions (5.6)]
- Impaired Wound Healing [see Warnings and Precautions (5.7)]
- Metabolic Disorders [see Warnings and Precautions (5.9)]
- Myelosuppression [see Warnings and Precautions (5.10)]
- Radiation Sensitization and Radiation Recall [see Warnings and Precautions (5.12)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, the adverse reaction rates observed cannot be directly compared to rates in other trials and may not reflect the rates observed in clinical practice.
Hormone Receptor-Positive, HER2-Negative Breast Cancer
The safety of everolimus (10 mg orally once daily) in combination with exemestane (25 mg orally once daily) (n=485) vs. placebo in combination with exemestane (n=239) was evaluated in a randomized, controlled trial (BOLERO-2) in patients with advanced or metastatic hormone receptor-positive, HER2-negative breast cancer. The median age of patients was 61 years (28 to 93 years), and 75% were white. The median follow-up was approximately 13 months.
The most common adverse reactions (incidence ≥30%) were stomatitis, infections, rash, fatigue, diarrhea, and decreased appetite. The most common Grade 3 to 4 adverse reactions (incidence ≥2%) were stomatitis, infections, hyperglycemia, fatigue, dyspnea, pneumonitis, and diarrhea. The most common laboratory abnormalities (incidence ≥50%) were hypercholesterolemia, hyperglycemia, increased aspartate transaminase (AST), anemia, leukopenia, thrombocytopenia, lymphopenia, increased alanine transaminase (ALT), and hypertriglyceridemia. The most common Grade 3 to 4 laboratory abnormalities (incidence ≥3%) were lymphopenia, hyperglycemia, anemia, hypokalemia, increased AST, increased ALT, and thrombocytopenia.
Fatal adverse reactions occurred in 2% of patients who received everolimus. The rate of adverse reactions resulting in permanent discontinuation was 24% for the everolimus arm. Dose adjustments (interruptions or reductions) occurred in 63% of patients in the everolimus arm.
Adverse reactions reported with an incidence of ≥10% for patients receiving everolimus vs. placebo are presented in Table 6. Laboratory abnormalities are presented in Table 7. The median duration of treatment with everolimus was 23.9 weeks; 33% were exposed to everolimus for a period of ≥32 weeks.
Table 6: Adverse Reactions Reported in ≥10% of Patients with Hormone Receptor-Positive Breast Cancer in BOLERO-2|
Everolimus with Exemestane N=482 |
Placebo with Exemestane N=238 | |||
|
All Grades % |
Grade 3 to 4 % |
All Grades % |
Grade 3 to 4 % | |
|
Gastrointestinal | ||||
|
Stomatitisa |
67 |
8d |
11 |
0.8 |
|
Diarrhea |
33 |
2 |
18 |
0.8 |
|
Nausea |
29 |
0.4 |
28 |
1 |
|
Vomiting |
17 |
1 |
12 |
0.8 |
|
Constipation |
14 |
0.4d |
13 |
0.4 |
|
Dry mouth |
11 |
0 |
7 |
0 |
|
General | ||||
|
Fatigue |
36 |
4 |
27 |
1d |
|
Edema peripheral |
19 |
1d |
6 |
0.4d |
|
Pyrexia |
15 |
0.2d |
7 |
0.4d |
|
Asthenia |
13 |
2 |
4 |
0 |
|
Infections | ||||
|
Infectionsb |
50 |
6 |
25 |
2d |
|
Investigations | ||||
|
Weight loss |
25 |
1d |
6 |
0 |
|
Metabolism and nutrition | ||||
|
Decreased appetite |
30 |
1d |
12 |
0.4d |
|
Hyperglycemia |
14 |
5 |
2 |
0.4d |
|
Musculoskeletal and connective tissue | ||||
|
Arthralgia |
20 |
0.8d |
17 |
0 |
|
Back pain |
14 |
0.2d |
10 |
0.8d |
|
Pain in extremity |
9 |
0.4d |
11 |
2d |
|
Nervous system | ||||
|
Dysgeusia |
22 |
0.2d |
6 |
0 |
|
Headache |
21 |
0.4d |
14 |
0 |
|
Psychiatric | ||||
|
Insomnia |
13 |
0.2d |
8 |
0 |
|
Respiratory, thoracic and mediastinal | ||||
|
Cough |
24 |
0.6d |
12 |
0 |
|
Dyspnea |
21 |
4 |
11 |
1 |
|
Epistaxis |
17 |
0 |
1 |
0 |
|
Pneumonitisc |
19 |
4 |
0.4 |
0 |
|
Skin and subcutaneous tissue | ||||
|
Rash |
39 |
1d |
6 |
0 |
|
Pruritus |
13 |
0.2d |
5 |
0 |
|
Alopecia |
10 |
0 |
5 |
0 |
|
Vascular | ||||
|
Hot flush |
6 |
0 |
14 |
0 |
|
Grading according to NCI CTCAE Version 3.0. | ||||
|
aIncludes stomatitis, mouth ulceration, aphthous stomatitis, glossodynia, gingival pain, glossitis, and lip ulceration. | ||||
|
bIncludes all reported infections, including but not limited to, urinary tract infections, respiratory tract (upper and lower) infections, skin infections, and gastrointestinal tract infections. | ||||
|
cIncludes pneumonitis, interstitial lung disease, lung infiltration, and pulmonary fibrosis. | ||||
|
dNo Grade 4 adverse reactions were reported. |
|
Laboratory Parameter |
Everolimus with Exemestane N=482 |
Placebo with Exemestane N=238 | ||
|
All Grades % |
Grade 3 to 4 % |
All Grades % |
Grade 3 to 4 % | |
|
Hematology****a | ||||
|
Anemia |
68 |
6 |
40 |
1 |
|
Leukopenia |
58 |
2b |
28 |
6 |
|
Thrombocytopenia |
54 |
3 |
5 |
0.4 |
|
Lymphopenia |
54 |
12 |
37 |
6 |
|
Neutropenia |
31 |
2b |
11 |
2 |
|
Chemistry | ||||
|
Hypercholesterolemia |
70 |
1 |
38 |
2 |
|
Hyperglycemia |
69 |
9 |
44 |
1 |
|
Increased AST |
69 |
4 |
45 |
3 |
|
Increased ALT |
51 |
4 |
29 |
5b |
|
Hypertriglyceridemia |
50 |
0.8b |
26 |
0 |
|
Hypoalbuminemia |
33 |
0.8b |
16 |
0.8b |
|
Hypokalemia |
29 |
4 |
7 |
1b |
|
Increased creatinine |
24 |
2 |
13 |
0 |
|
Grading according to NCI CTCAE Version 3.0. | ||||
|
aReflects corresponding adverse drug reaction reports of anemia, leukopenia, lymphopenia, neutropenia, and thrombocytopenia (collectively as pancytopenia), which occurred at lower frequency. | ||||
|
bNo Grade 4 laboratory abnormalities were reported. |
Topical Prophylaxis for Stomatitis
In a single arm study (SWISH; N=92) in postmenopausal women with hormone
receptor-positive,
HER2-negative breast cancer beginning everolimus (10 mg orally once daily) in
combination with exemestane (25 mg orally once daily), patients started
dexamethasone 0.5 mg/5 mL alcohol-free mouthwash (10 mL swished for 2 minutes
and spat, 4 times daily for 8 weeks) concurrently with everolimus and
exemestane. No food or drink was to be consumed for at least 1 hour after
swishing and spitting the dexamethasone mouthwash. The primary objective of
this study was to assess the incidence of Grade 2 to 4 stomatitis within 8
weeks. The incidence of Grade 2 to 4 stomatitis within 8 weeks was 2%, which
was lower than the 33% reported in the BOLERO-2 trial. The incidence of Grade
1 stomatitis was 19%. No cases of Grade 3 or 4 stomatitis were reported. Oral
candidiasis was reported in 2% of patients in this study compared to 0.2% in
the BOLERO-2 trial.
Coadministration of everolimus and dexamethasone alcohol-free oral solution has not been studied in pediatric patients.
Pancreatic Neuroendocrine Tumors (PNET)
In a randomized, controlled trial (RADIANT-3) of everolimus (n=204) vs. placebo (n=203) in patients with advanced PNET the median age of patients was 58 years (20 to 87 years), 79% were white, and 55% were male. Patients on the placebo arm could cross over to open-label everolimus upon disease progression.
The most common adverse reactions (incidence ≥30%) were stomatitis, rash, diarrhea, fatigue, edema, abdominal pain, nausea, fever, and headache. The most common Grade 3 to 4 adverse reactions (incidence ≥5%) were stomatitis and diarrhea. The most common laboratory abnormalities (incidence ≥50%) were anemia, hyperglycemia, increased alkaline phosphatase, hypercholesterolemia, decreased bicarbonate, and increased AST. The most common Grade 3 to 4 laboratory abnormalities (incidence ≥3%) were hyperglycemia, lymphopenia, anemia, hypophosphatemia, increased alkaline phosphatase, neutropenia, increased AST, hypokalemia, and thrombocytopenia.
Deaths during double-blind treatment where an adverse reaction was the primary cause occurred in seven patients on everolimus. Causes of death on the everolimus arm included one case of each of the following: acute renal failure, acute respiratory distress, cardiac arrest, death (cause unknown), hepatic failure, pneumonia, and sepsis. After cross-over to open-label everolimus, there were three additional deaths, one due to hypoglycemia and cardiac arrest in a patient with insulinoma, one due to myocardial infarction with congestive heart failure, and the other due to sudden death. The rate of adverse reactions resulting in permanent discontinuation was 20% for the everolimus group. Dose delay or reduction was necessary in 61% of everolimus patients. Grade 3 to 4 renal failure occurred in six patients in the everolimus arm. Thrombotic events included five patients with pulmonary embolus in the everolimus arm as well as three patients with thrombosis in the everolimus arm.
Table 8 compares the incidence of adverse reactions reported with an incidence of ≥10% for patients receiving everolimus vs. placebo. Laboratory abnormalities are summarized in Table 9. The median duration of treatment in patients who received everolimus was 37 weeks.
In female patients aged 18 to 55 years, irregular menstruation occurred in 5 of 46 (11%) everolimus-treated females.
Table 8: Adverse Reactions Reported in ≥10% of Patients with PNET in RADIANT-3|
Everolimus N=204 |
Placebo N=203 | |||
|
All Grades % |
Grade 3 to 4 % |
All Grades % |
Grade 3 to 4 % | |
|
Gastrointestinal | ||||
|
Stomatitisa |
70 |
7d |
20 |
0 |
|
Diarrheab |
50 |
6 |
25 |
3d |
|
Abdominal pain |
36 |
4d |
32 |
7 |
|
Nausea |
32 |
2d |
33 |
2d |
|
Vomiting |
29 |
1d |
21 |
2d |
|
Constipation |
14 |
0 |
13 |
0.5d |
|
Dry mouth |
11 |
0 |
4 |
0 |
|
General | ||||
|
Fatigue/malaise |
45 |
4 |
27 |
3 |
|
Edema (general and peripheral) |
39 |
2 |
12 |
1d |
|
Fever |
31 |
1 |
13 |
0.5d |
|
Asthenia |
19 |
3d |
20 |
3d |
|
Infections | ||||
|
Nasopharyngitis/rhinitis/URI |
25 |
0 |
13 |
0 |
|
Urinary tract infection |
16 |
0 |
6 |
0.5d |
|
Investigations | ||||
|
Weight loss |
28 |
0.5d |
11 |
0 |
|
Metabolism and nutrition | ||||
|
Decreased appetite |
30 |
1d |
18 |
1d |
|
Diabetes mellitus |
10 |
2d |
0.5 |
0 |
|
Musculoskeletal and connective tissue | ||||
|
Arthralgia |
15 |
1 |
7 |
0.5d |
|
Back pain |
15 |
1d |
11 |
1d |
|
Pain in extremity |
14 |
0.5d |
6 |
1d |
|
Muscle spasms |
10 |
0 |
4 |
0 |
|
Nervous system | ||||
|
Headache/migraine |
30 |
0.5d |
15 |
1d |
|
Dysgeusia |
19 |
0 |
5 |
0 |
|
Dizziness |
12 |
0.5d |
7 |
0 |
|
Psychiatric | ||||
|
Insomnia |
14 |
0 |
8 |
0 |
|
Respiratory, thoracic and mediastinal | ||||
|
Cough/productive cough |
25 |
0.5d |
13 |
0 |
|
Epistaxis |
22 |
0 |
1 |
0 |
|
Dyspnea/dyspnea exertional |
20 |
3 |
7 |
0.5d |
|
Pneumonitisc |
17 |
4 |
0 |
0 |
|
Oropharyngeal pain |
11 |
0 |
6 |
0 |
|
Skin and subcutaneous | ||||
|
Rash |
59 |
0.5 |
19 |
0 |
|
Nail disorders |
22 |
0.5 |
2 |
0 |
|
Pruritus/pruritus generalized |
21 |
0 |
13 |
0 |
|
Dry skin/xeroderma |
13 |
0 |
6 |
0 |
|
Vascular | ||||
|
Hypertension |
13 |
1 |
6 |
1d |
|
Grading according to NCI CTCAE Version 3.0. | ||||
|
aIncludes stomatitis, aphthous stomatitis, gingival pain/swelling/ulceration, glossitis, glossodynia, lip ulceration, mouth ulceration, tongue ulceration, and mucosal inflammation. | ||||
|
bIncludes diarrhea, enteritis, enterocolitis, colitis, defecation urgency, and steatorrhea. | ||||
|
cIncludes pneumonitis, interstitial lung disease, pulmonary fibrosis, and restrictive pulmonary disease. | ||||
|
dNo Grade 4 adverse reactions were reported. |
|
Laboratory parameter |
Everolimus N=204 |
Placebo N=203 | ||
|
All Grades % |
Grade 3 to 4 % |
All Grades % |
Grade 3 to 4 % | |
|
Hematology | ||||
|
Anemia |
86 |
15 |
63 |
1 |
|
Lymphopenia |
45 |
16 |
22 |
4 |
|
Thrombocytopenia |
45 |
3 |
11 |
0 |
|
Leukopenia |
43 |
2 |
13 |
0 |
|
Neutropenia |
30 |
4 |
17 |
2 |
|
Chemistry | ||||
|
Hyperglycemia (fasting) |
75 |
17 |
53 |
6 |
|
Increased alkaline phosphatase |
74 |
8 |
66 |
8 |
|
Hypercholesterolemia |
66 |
0.5 |
22 |
0 |
|
Bicarbonate decreased |
56 |
0 |
40 |
0 |
|
Increased AST |
56 |
4 |
41 |
4 |
|
Increased ALT |
48 |
2 |
35 |
2 |
|
Hypophosphatemia |
40 |
10 |
14 |
3 |
|
Hypertriglyceridemia |
39 |
0 |
10 |
0 |
|
Hypocalcemia |
37 |
0.5 |
12 |
0 |
|
Hypokalemia |
23 |
4 |
5 |
0 |
|
Increased creatinine |
19 |
2 |
14 |
0 |
|
Hyponatremia |
16 |
1 |
16 |
1 |
|
Hypoalbuminemia |
13 |
1 |
8 |
0 |
|
Hyperbilirubinemia |
10 |
1 |
14 |
2 |
|
Hyperkalemia |
7 |
0 |
10 |
0.5 |
|
Grading according to NCI CTCAE Version 3.0. |
Neuroendocrine Tumors (NET) of Gastrointestinal (GI) or Lung Origin
In a randomized, controlled trial (RADIANT-4) of everolimus (n=202 treated) vs. placebo (n=98 treated) in patients with advanced non-functional NET of GI or lung origin, the median age of patients was 63 years (22 to 86 years), 76% were white, and 53% were female. The median duration of exposure to everolimus was 9.3 months; 64% of patients were treated for ≥6 months and 39% were treated for ≥12 months. Everolimus was discontinued for adverse reactions in 29% of patients, dose reduction or delay was required in 70% of everolimus- treated patients.
Serious adverse reactions occurred in 42% of everolimus-treated patients and included 3 fatal events (cardiac failure, respiratory failure, and septic shock). Adverse reactions occurring at an incidence of ≥10% and at ≥5% absolute incidence over placebo (all Grades) or ≥2% higher incidence over placebo (Grade 3 and 4) are presented in Table 10. Laboratory abnormalities are presented in Table 11.
Table 10: Adverse Reactions in ≥10% of Everolimus-Treated Patients with Non-Functional NET of GI or Lung Origin in RADIANT-4|
Everolimus N=202 |
Placebo N=98 | |||
|
All Grades % |
Grade 3 to 4 % |
All Grades % |
Grade 3 to 4 % | |
|
Gastrointestinal | ||||
|
Stomatitisa |
63 |
9d |
22 |
0 |
|
Diarrhea |
41 |
9 |
31 |
2d |
|
Nausea |
26 |
3 |
17 |
1d |
|
Vomiting |
15 |
4d |
12 |
2d |
|
General | ||||
|
Peripheral edema |
39 |
3d |
6 |
1d |
|
Fatigue |
37 |
5 |
36 |
1d |
|
Asthenia |
23 |
3 |
8 |
0 |
|
Pyrexia |
23 |
2 |
8 |
0 |
|
Infections | ||||
|
Infectionsb |
58 |
11 |
29 |
2 |
|
Investigations | ||||
|
Weight loss |
22 |
2d |
11 |
1d |
|
Metabolism and nutrition | ||||
|
Decreased appetite |
22 |
1d |
17 |
1d |
|
Nervous system | ||||
|
Dysgeusia |
18 |
1d |
4 |
0 |
|
Respiratory, thoracic and mediastinal | ||||
|
Cough |
27 |
0 |
20 |
0 |
|
Dyspnea |
20 |
3d |
11 |
2 |
|
Pneumonitisc |
16 |
2d |
2 |
0 |
|
Epistaxis |
13 |
1d |
3 |
0 |
|
Skin and subcutaneous | ||||
|
Rash |
30 |
1d |
9 |
0 |
|
Pruritus |
17 |
1d |
9 |
0 |
|
Grading according to NCI CTCAE Version 4.03 | ||||
|
aIncludes stomatitis, mouth ulceration, aphthous stomatitis, gingival pain, glossitis, tongue ulceration, and mucosal inflammation. | ||||
|
bUrinary tract infection, nasopharyngitis, upper respiratory tract infection, lower respiratory tract infection (pneumonia, bronchitis), abscess, pyelonephritis, septic shock and viral myocarditis. | ||||
|
cIncludes pneumonitis and interstitial lung disease. | ||||
|
dNo Grade 4 adverse reactions were reported. |
|
Everolimus N=202 |
Placebo N=98 | |||
|
All Grades % |
Grade 3 to 4 % |
All Grades % |
Grade 3 to 4 % | |
|
Hematology | ||||
|
Anemia |
81 |
5a |
41 |
2a |
|
Lymphopenia |
66 |
16 |
32 |
2a |
|
Leukopenia |
49 |
2a |
17 |
0 |
|
Thrombocytopenia |
33 |
2 |
11 |
0 |
|
Neutropenia |
32 |
2a |
15 |
3a |
|
Chemistry | ||||
|
Hypercholesterolemia |
71 |
0 |
37 |
0 |
|
Increased AST |
57 |
2 |
34 |
2a |
|
Hyperglycemia (fasting) |
55 |
6a |
36 |
1a |
|
Increased ALT |
46 |
5 |
39 |
1a |
|
Hypophosphatemia |
43 |
4a |
15 |
2a |
|
Hypertriglyceridemia |
30 |
3 |
8 |
1a |
|
Hypokalemia |
27 |
6 |
12 |
3a |
|
Hypoalbuminemia |
18 |
0 |
8 |
0 |
|
Grading according to NCI CTCAE Version 4.03. | ||||
|
aNo Grade 4 laboratory abnormalities were reported. |
Renal Cell Carcinoma (RCC)
The data described below reflect exposure to everolimus (n=274) and placebo (n=137) in a randomized, controlled trial (RECORD-1) in patients with metastatic RCC who received prior treatment with sunitinib and/or sorafenib. The median age of patients was 61 years (27 to 85 years), 88% were white, and 78% were male. The median duration of blinded study treatment was 141 days (19 to 451 days) for patients receiving everolimus.
The most common adverse reactions (incidence ≥30%) were stomatitis, infections, asthenia, fatigue, cough, and diarrhea. The most common Grade 3 to 4 adverse reactions (incidence ≥3%) were infections, dyspnea, fatigue, stomatitis, dehydration, pneumonitis, abdominal pain, and asthenia. The most common laboratory abnormalities (incidence ≥ 50%) were anemia, hypercholesterolemia, hypertriglyceridemia, hyperglycemia, lymphopenia, and increased creatinine. The most common Grade 3 to 4 laboratory abnormalities (incidence ≥3%) were lymphopenia, hyperglycemia, anemia, hypophosphatemia, and hypercholesterolemia.
Deaths due to acute respiratory failure (0.7%), infection (0.7%), and acute renal failure (0.4%) were observed on the everolimus arm. The rate of adverse reactions resulting in permanent discontinuation was 14% for the everolimus group. The most common adverse reactions leading to treatment discontinuation were pneumonitis and dyspnea. Infections, stomatitis, and pneumonitis were the most common reasons for treatment delay or dose reduction. The most common medical interventions required during everolimus treatment were for infections, anemia, and stomatitis.
Adverse reactions reported with an incidence of ≥10% for patients receiving everolimus vs. placebo are presented in Table 12. Laboratory abnormalities are presented in Table 13.
Table 12: Adverse Reactions Reported in ≥10% of Patients with RCC and at a Higher Rate in the Everolimus Arm than in the Placebo Arm in RECORD-1|
Everolimus N=274 |
Placebo N=137 | |||
|
All Grades % |
Grade 3 to 4 % |
All Grades % |
Grade 3 to 4 % | |
|
Gastrointestinal | ||||
|
Stomatitisa |
44 |
4 |
8 |
0 |
|
Diarrhea |
30 |
2d |
7 |
0 |
|
Nausea |
26 |
2d |
19 |
0 |
|
Vomiting |
20 |
2d |
12 |
0 |
|
Infections****b |
37 |
10 |
18 |
2 |
|
General | ||||
|
Asthenia |
33 |
4 |
23 |
4 |
|
Fatigue |
31 |
6d |
27 |
4 |
|
Edema peripheral |
25 |
<1d |
8 |
<1d |
|
Pyrexia |
20 |
<1d |
9 |
0 |
|
Mucosal inflammation |
19 |
2d |
1 |
0 |
|
Respiratory, thoracic and mediastinal | ||||
|
Cough |
30 |
<1d |
16 |
0 |
|
Dyspnea |
24 |
8 |
15 |
3d |
|
Epistaxis |
18 |
0 |
0 |
0 |
|
Pneumonitisc |
14 |
4d |
0 |
0 |
|
Skin and subcutaneous tissue | ||||
|
Rash |
29 |
1d |
7 |
0 |
|
Pruritus |
14 |
<1d |
7 |
0 |
|
Dry skin |
13 |
<1d |
5 |
0 |
|
Metabolism and nutrition | ||||
|
Anorexia |
25 |
2d |
14 |
<1d |
|
Nervous system | ||||
|
Headache |
19 |
1 |
9 |
<1d |
|
Dysgeusia |
10 |
0 |
2 |
0 |
|
Musculoskeletal and connective tissue | ||||
|
Pain in extremity |
10 |
1d |
7 |
0 |
|
Grading according to NCI CTCAE Version 3.0. | ||||
|
aStomatitis (including aphthous stomatitis), and mouth and tongue ulceration. | ||||
|
bIncludes all reported infections including, but not limited to, respiratory tract (upper and lower) infections, urinary tract infections, and skin infections. | ||||
|
cIncludes pneumonitis, interstitial lung disease, lung infiltration, pulmonary alveolar hemorrhage, pulmonary toxicity, and alveolitis. | ||||
|
dNo Grade 4 adverse reactions were reported. |
Other notable adverse reactions occurring more frequently with everolimus than with placebo, but with an incidence of <10% include:
Gastrointestinal: Abdominal pain (9%), dry mouth (8%), hemorrhoids (5%), dysphagia (4%)
General: Weight loss (9%), chest pain (5%), chills (4%), impaired wound healing (<1%)
Respiratory, thoracic and mediastinal: Pleural effusion (7%), pharyngolaryngeal pain (4%), rhinorrhea (3%)
Skin and subcutaneous tissue: Hand-foot syndrome (reported as palmar-plantar erythrodysesthesia syndrome) (5%), nail disorder (5%), erythema (4%), onychoclasis (4%), skin lesion (4%), acneiform dermatitis (3%), angioedema (<1%)
Metabolism and nutrition: Exacerbation of pre-existing diabetes mellitus (2%), new onset of diabetes mellitus (<1%)
Psychiatric: Insomnia (9%)
Nervous system: Dizziness (7%), paresthesia (5%)
Ocular: Eyelid edema (4%), conjunctivitis (2%)
Vascular: Hypertension (4%), deep vein thrombosis (<1%)
Renal and urinary: Renal failure (3%)
Cardiac: Tachycardia (3%), congestive cardiac failure (1%)
Musculoskeletal and connective tissue: Jaw pain (3%)
Hematologic: Hemorrhage (3%)
Table 13: Selected Laboratory Abnormalities Reported in Patients with RCC at a Higher Rate in the Everolimus Arm than the Placebo Arm in RECORD-1|
Laboratory parameter |
Everolimus N=274 |
Placebo N=137 | ||
|
All Grades % |
Grade 3 to 4 % |
All Grades % |
Grade 3 to 4 % | |
|
Hematology****a | ||||
|
Anemia |
92 |
13 |
79 |
6 |
|
Lymphopenia |
51 |
18 |
28 |
5b |
|
Thrombocytopenia |
23 |
1b |
2 |
<1 |
|
Neutropenia |
14 |
<1 |
4 |
0 |
|
Chemistry | ||||
|
Hypercholesterolemia |
77 |
4b |
35 |
0 |
|
Hypertriglyceridemia |
73 |
<1b |
34 |
0 |
|
Hyperglycemia |
57 |
16 |
25 |
2b |
|
Increased creatinine |
50 |
2b |
34 |
0 |
|
Hypophosphatemia |
37 |
6b |
8 |
0 |
|
Increased AST |
25 |
1 |
7 |
0 |
|
Increased ALT |
21 |
1b |
4 |
0 |
|
Hyperbilirubinemia |
3 |
1 |
2 |
0 |
|
Grading according to NCI CTCAE Version 3.0. | ||||
|
aReflects corresponding adverse drug reaction reports of anemia, leukopenia, lymphopenia, neutropenia, and thrombocytopenia (collectively pancytopenia), which occurred at lower frequency. | ||||
|
bNo Grade 4 laboratory abnormalities were reported. |
Tuberous Sclerosis Complex (TSC)-Associated Renal Angiomyolipoma
The data described below are based on a randomized (2:1), double-blind, placebo-controlled trial (EXIST-2) of everolimus in 118 patients with renal angiomyolipoma as a feature of TSC (n=113) or sporadic lymphangioleiomyomatosis (n=5). The median age of patients was 31 years (18 to 61 years), 89% were white, and 34% were male. The median duration of blinded study treatment was 48 weeks (2 to 115 weeks) for patients receiving everolimus.
The most common adverse reaction reported for everolimus (incidence ≥30%) was stomatitis. The most common Grade 3 to 4 adverse reactions (incidence ≥2%) were stomatitis and amenorrhea. The most common laboratory abnormalities (incidence ≥50%) were hypercholesterolemia, hypertriglyceridemia, and anemia. The most common Grade 3 to 4 laboratory abnormality (incidence ≥3%) was hypophosphatemia.
The rate of adverse reactions resulting in permanent discontinuation was 3.8% in the everolimus-treated patients. Adverse reactions leading to permanent discontinuation in the everolimus arm were hypersensitivity/angioedema/bronchospasm, convulsion, and hypophosphatemia. Dose adjustments (interruptions or reductions) due to adverse reactions occurred in 52% of everolimus-treated patients. The most common adverse reaction leading to everolimus dose adjustment was stomatitis.
Adverse reactions reported with an incidence of ≥10% for patients receiving everolimus and occurring more frequently with everolimus than with placebo are presented in Table 14. Laboratory abnormalities are presented in Table 15.
Table 14: Adverse Reactions Reported in ≥10% of Everolimus-Treated Patients with TSC-Associated Renal Angiomyolipoma in EXIST-2|
Everolimus N=79 |
Placebo N=39 | |||
|
All Grades % |
Grade 3 to 4 % |
All Grades % |
Grade 3 to 4 % | |
|
Gastrointestinal | ||||
|
Stomatitisa |
78 |
6b |
23 |
0 |
|
Vomiting |
15 |
0 |
5 |
0 |
|
Diarrhea |
14 |
0 |
5 |
0 |
|
General | ||||
|
Peripheral edema |
13 |
0 |
8 |
0 |
|
Infections | ||||
|
Upper respiratory tract infection |
11 |
0 |
5 |
0 |
|
Musculoskeletal and connective tissue | ||||
|
Arthralgia |
13 |
0 |
5 |
0 |
|
Respiratory, thoracic and mediastinal | ||||
|
Cough |
20 |
0 |
13 |
0 |
|
Skin and subcutaneous tissue | ||||
|
Acne |
22 |
0 |
5 |
0 |
|
Grading according to NCI CTCAE Version 3.0. | ||||
|
aIncludes stomatitis, aphthous stomatitis, mouth ulceration, gingival pain, glossitis, and glossodynia. | ||||
|
bNo Grade 4 adverse reactions were reported. |
Amenorrhea occurred in 15% of everolimus-treated females (8 of 52). Other adverse reactions involving the female reproductive system were menorrhagia (10%), menstrual irregularities (10%), and vaginal hemorrhage (8%).
The following additional adverse reactions occurred in less than 10% of everolimus-treated patients: epistaxis (9%), decreased appetite (6%), otitis media (6%), depression (5%), abnormal taste (5%), increased blood luteinizing hormone (LH) levels (4%), increased blood follicle stimulating hormone (FSH) levels (3%), hypersensitivity (3%), ovarian cyst (3%), pneumonitis (1%), and angioedema (1%).
Table 15: Selected Laboratory Abnormalities Reported in Everolimus- Treated Patients with TSC-Associated Renal Angiomyolipoma in EXIST-2|
Everolimus N=79 |
Placebo N=39 | |||
|
All Grades % |
Grade 3 to 4 % |
All Grades % |
Grade 3 to 4 % | |
|
Hematology | ||||
|
Anemia |
61 |
0 |
49 |
0 |
|
Leukopenia |
37 |
0 |
21 |
0 |
|
Neutropenia |
25 |
1 |
26 |
0 |
|
Lymphopenia |
20 |
1a |
8 |
0 |
|
Thrombocytopenia |
19 |
0 |
3 |
0 |
|
Chemistry | ||||
|
Hypercholesterolemia |
85 |
1a |
46 |
0 |
|
Hypertriglyceridemia |
52 |
0 |
10 |
0 |
|
Hypophosphatemia |
49 |
5a |
15 |
0 |
|
Increased alkaline phosphatase |
32 |
1a |
10 |
0 |
|
Increased AST |
23 |
1a |
8 |
0 |
|
Increased ALT |
20 |
1a |
15 |
0 |
|
Hyperglycemia (fasting) |
14 |
0 |
8 |
0 |
|
Grading according to NCI CTCAE Version 3.0. | ||||
|
aNo Grade 4 laboratory abnormalities were reported. |
Updated safety information from 112 patients treated with everolimus for a median duration of 3.9 years identified the following additional adverse reactions and selected laboratory abnormalities: increased partial thromboplastin time (63%), increased prothrombin time (40%), decreased fibrinogen (38%), urinary tract infection (31%), proteinuria (18%), abdominal pain (16%), pruritus (12%), gastroenteritis (12%), myalgia (11%), and pneumonia (10%).
TSC-Associated Subependymal Giant Cell Astrocytoma (SEGA)
The data described below are based on a randomized (2:1), double-blind, placebo-controlled trial (EXIST-1) of everolimus in 117 patients with SEGA and TSC. The median age of patients was 9.5 years (0.8 to 26 years), 93% were white, and 57% were male. The median duration of blinded study treatment was 52 weeks (24 to 89 weeks) for patients receiving everolimus.
The most common adverse reactions reported for everolimus (incidence ≥30%) were stomatitis and respiratory tract infection. The most common Grade 3 to 4 adverse reactions (incidence ≥2%) were stomatitis, pyrexia, pneumonia, gastroenteritis, aggression, agitation, and amenorrhea. The most common laboratory abnormalities (incidence ≥50%) were hypercholesterolemia and elevated partial thromboplastin time. The most common Grade 3 to 4 laboratory abnormality (incidence ≥3%) was neutropenia.
There were no adverse reactions resulting in permanent discontinuation. Dose adjustments (interruptions or reductions) due to adverse reactions occurred in 55% of everolimus-treated patients. The most common adverse reaction leading to everolimus dose adjustment was stomatitis.
Adverse reactions reported with an incidence of ≥10% for patients receiving everolimus and occurring more frequently with everolimus than with placebo are reported in Table 16. Laboratory abnormalities are presented in Table 17.
Table 16: Adverse Reactions Reported in ≥10% of Everolimus-Treated Patients with TSC-Associated SEGA in EXIST-1|
Everolimus N=78 |
Placebo N=39 | |||
|
All Grades % |
Grade 3 to 4 % |
All Grades % |
Grade 3 to 4 % | |
|
Gastrointestinal | ||||
|
Stomatitisa |
62 |
9f |
26 |
3f |
|
Vomiting |
22 |
1f |
13 |
0 |
|
Diarrhea |
17 |
0 |
5 |
0 |
|
Constipation |
10 |
0 |
3 |
0 |
|
Infections | ||||
|
Respiratory tract infectionb |
31 |
3 |
23 |
0 |
|
Gastroenteritisc |
10 |
5 |
3 |
0 |
|
Pharyngitis streptococcal |
10 |
0 |
3 |
0 |
|
General | ||||
|
Pyrexia |
23 |
6f |
18 |
3f |
|
Fatigue |
14 |
0 |
3 |
0 |
|
Psychiatric | ||||
|
Anxiety, aggression or other behavioral disturbanced |
21 |
5f |
3 |
0 |
|
Skin and subcutaneous tissue | ||||
|
Rashe |
21 |
0 |
8 |
0 |
|
Acne |
10 |
0 |
5 |
0 |
|
Grading according to NCI CTCAE Version 3.0. | ||||
|
aIncludes mouth ulceration, stomatitis, and lip ulceration. | ||||
|
bIncludes respiratory tract infection, upper respiratory tract infection, and respiratory tract infection viral. | ||||
|
cIncludes gastroenteritis, gastroenteritis viral, and gastrointestinal infection. | ||||
|
dIncludes agitation, anxiety, panic attack, aggression, abnormal behavior, and obsessive compulsive disorder. | ||||
|
eIncludes rash, rash generalized, rash macular, rash maculo-papular, rash papular, dermatitis allergic, and urticaria. | ||||
|
fNo Grade 4 adverse reactions were reported. |
Amenorrhea occurred in 17% of everolimus-treated females aged 10 to 55 years (3 of 18). For this same group of everolimus-treated females, the following menstrual abnormalities were reported: dysmenorrhea (6%), menorrhagia (6%), metrorrhagia (6%), and unspecified menstrual irregularity (6%).
The following additional adverse reactions occurred in less than 10% of everolimus-treated patients: nausea (8%), pain in extremity (8%), insomnia (6%), pneumonia (6%), epistaxis (5%), hypersensitivity (3%), increased blood luteinizing hormone (LH) levels (1%), and pneumonitis (1%).
Table 17: Selected Laboratory Abnormalities Reported in Everolimus- Treated Patients with TSC-Associated SEGA in EXIST-1|
Everolimus N=78 |
Placebo N=39 | |||
|
All Grades % |
Grade 3 to 4 % |
All Grades % |
Grade 3 to 4 % | |
|
Hematology | ||||
|
Elevated partial thromboplastin time |
72 |
3a |
44 |
5a |
|
Neutropenia |
46 |
9a |
41 |
3a |
|
Anemia |
41 |
0 |
21 |
0 |
|
Chemistry | ||||
|
Hypercholesterolemia |
81 |
0 |
39 |
0 |
|
Elevated AST |
33 |
0 |
0 |
0 |
|
Hypertriglyceridemia |
27 |
0 |
15 |
0 |
|
Elevated ALT |
18 |
0 |
3 |
0 |
|
Hypophosphatemia |
9 |
1a |
3 |
0 |
|
Grading according to NCI CTCAE Version 3.0. | ||||
|
aNo Grade 4 laboratory abnormalities were reported. |
Updated safety information from 111 patients treated with everolimus for a median duration of 47 months identified the following additional notable adverse reactions and selected laboratory abnormalities: decreased appetite (14%), hyperglycemia (13%), hypertension (11%), urinary tract infection (9%), decreased fibrinogen (8%), cellulitis (6%), abdominal pain (5%), decreased weight (5%), elevated creatinine (5%), and azoospermia (1%).
6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of everolimus. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate frequency or establish a causal relationship to drug exposure:
- Blood and lymphatic disorders: Thrombotic microangiopathy
- Cardiac: Cardiac failure with some cases reported with pulmonary hypertension (including pulmonary arterial hypertension) as a secondary event
- Gastrointestinal: Acute pancreatitis
- Hepatobiliary: Cholecystitis and cholelithiasis
- Infections: Sepsis and septic shock
- Nervous system: Reflex sympathetic dystrophy
- Vascular: Arterial thrombotic events, lymphedema
- Injury, poisoning and procedural complications: Radiation Sensitization and Radiation Recall
- Breast cancer, NET, RCC: Most common adverse reactions (incidence ≥30%) include stomatitis, infections, rash, fatigue, diarrhea, edema, abdominal pain, nausea, fever, asthenia, cough, headache, and decreased appetite. (6.1)
- TSC-Associated Renal Angiomyolipoma: Most common adverse reaction (incidence ≥30%) is stomatitis. (6.1)
- TSC-Associated SEGA: Most common adverse reactions (incidence ≥30%) are stomatitis and respiratory tract infection. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Teva at 1-888-838-2872 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
7.1 Effect of Other Drugs on Everolimus
Inhibitors
Avoid the concomitant use of P-gp and strong CYP3A4 inhibitors [see Dosage and Administration (2.11), Clinical Pharmacology (12.3)].
Reduce the dose for patients taking everolimus with a P-gp and moderate CYP3A4 inhibitor as recommended [see Dosage and Administration (2.11), Clinical Pharmacology (12.3)].
Inducers
Increase the dose for patients taking everolimus with a P-gp and strong CYP3A4 inducer as recommended [see Dosage and Administration (2.12), Clinical Pharmacology (12.3)].
7.2 Effects of Combination Use of Angiotensin Converting Enzyme (ACE)
Inhibitors
Patients taking concomitant ACE inhibitors with everolimus may be at increased risk for angioedema. Avoid the concomitant use of ACE inhibitors with everolimus [see Warnings and Precautions (5.4)].
- P-gp and strong CYP3A4 inhibitors: Avoid concomitant use. (2.11, 7.1)
- P-gp and moderate CYP3A4 inhibitors: Reduce the dose as recommended. (2.11, 7.1)
- P-gp and strong CYP3A4 inducers: Increase the dose as recommended. (2.12, 7.1)
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Everolimus is an inhibitor of mammalian target of rapamycin (mTOR), a serine-
threonine kinase, downstream of the PI3K/AKT pathway. The mTOR pathway is
dysregulated in several human cancers and in tuberous sclerosis complex (TSC).
Everolimus binds to an intracellular protein, FKBP-12, resulting in an
inhibitory complex formation with mTOR complex 1 (mTORC1) and thus inhibition
of mTOR kinase activity. Everolimus reduced the activity of S6 ribosomal
protein kinase (S6K1) and eukaryotic initiation factor 4E-binding protein
(4E-BP1), downstream effectors of mTOR, involved in protein synthesis. S6K1 is
a substrate of mTORC1 and phosphorylates the activation domain 1 of the
estrogen receptor which results in ligand-independent activation of the
receptor. In addition, everolimus inhibited the expression of
hypoxia-inducible factor (e.g., HIF-1) and reduced the expression of vascular
endothelial growth factor (VEGF). Inhibition of mTOR by everolimus has been
shown to reduce cell proliferation, angiogenesis, and glucose uptake in in
vitro and/or in vivo studies.
Constitutive activation of the PI3K/Akt/mTOR pathway can contribute to endocrine resistance in breast cancer. In vitro studies show that estrogen- dependent and HER2+ breast cancer cells are sensitive to the inhibitory effects of everolimus, and that combination treatment with everolimus and Akt, HER2, or aromatase inhibitors enhances the anti-tumor activity of everolimus in a synergistic manner.
Two regulators of mTORC1 signaling are the oncogene suppressors tuberin- sclerosis complexes 1 and 2 (TSC1, TSC2). Loss or inactivation of either TSC1 or TSC2 leads to activation of downstream signaling. In TSC, a genetic disorder, inactivating mutations in either the TSC1 or the TSC2 gene lead to hamartoma formation throughout the body as well as seizures and epileptogenesis. Overactivation of mTOR results in neuronal dysplasia, aberrant axonogenesis and dendrite formation, increased excitatory synaptic currents, reduced myelination, and disruption of the cortical laminar structure causing abnormalities in neuronal development and function. Treatment with an mTOR inhibitor in animal models of mTOR dysregulation in the brain resulted in seizure suppression, prevention of the development of new- onset seizures, and prevention of premature death.
12.2 Pharmacodynamics
Exposure-Response Relationship
In patients with TSC-associated subependymal giant cell astrocytoma (SEGA), the magnitude of the reduction in SEGA volume was correlated with the everolimus trough concentration.
Cardiac Electrophysiology
In a randomized, placebo-controlled, cross-over study, 59 healthy subjects were administered a single oral dose of everolimus (20 mg and 50 mg) and placebo. Everolimus at single doses up to 50 mg did not prolong the QT/QTc interval.
12.3 Pharmacokinetics
Absorption
After administration of everolimus in patients with advanced solid tumors, peak everolimus concentrations are reached 1 to 2 hours after administration of oral doses ranging from 5 mg to 70 mg. Following single doses, Cmax is dose-proportional with daily dosing between 5 mg and 10 mg. With single doses of 20 mg and higher, the increase in Cmax is less than dose-proportional; however, AUC shows dose-proportionality over the 5 mg to 70 mg dose range. Steady-state was achieved within 2 weeks following once-daily dosing.
In patients with TSC-associated SEGA, everolimus Cmin was approximately dose- proportional within the dose range from 1.35 mg/m2 to 14.4 mg/m2.
Effect of Food: In healthy subjects, a high-fat meal (containing approximately 1,000 calories and 55 grams of fat) reduced systemic exposure to everolimus 10 mg (as measured by AUC) by 22% and the peak blood concentration Cmax by 54%. Light-fat meals (containing approximately 500 calories and 20 grams of fat) reduced AUC by 32% and Cmax by 42%.
Distribution
The blood-to-plasma ratio of everolimus, which is concentration-dependent over the range of 5 to 5,000 ng/mL, is 17% to 73%. The amount of everolimus confined to the plasma is approximately 20% at blood concentrations observed in cancer patients given everolimus 10 mg orally once daily. Plasma protein binding is approximately 74% both in healthy subjects and in patients with moderate hepatic impairment.
Elimination
The mean elimination half-life of everolimus is approximately 30 hours.
Metabolism: Everolimus is a substrate of CYP3A4. Following oral administration, everolimus is the main circulating component in human blood. Six main metabolites of everolimus have been detected in human blood, including three monohydroxylated metabolites, two hydrolytic ring-opened products, and a phosphatidylcholine conjugate of everolimus. These metabolites were also identified in animal species used in toxicity studies, and showed approximately 100-times less activity than everolimus itself.
Excretion: No specific elimination studies have been undertaken in cancer patients. Following the administration of a 3 mg single dose of radiolabeled everolimus in patients who were receiving cyclosporine, 80% of the radioactivity was recovered from the feces, while 5% was excreted in the urine. The parent substance was not detected in urine or feces.
Specific Populations
No relationship was apparent between oral clearance and age or sex in patients with cancer.
Patients with Renal Impairment: No significant influence of creatinine clearance (25 to 178 mL/min) was detected on oral clearance (CL/F) of everolimus.
Patients with Hepatic Impairment: Compared to normal subjects, there was a 1.8-fold, 3.2-fold, and 3.6-fold increase in AUC for subjects with mild (Child-Pugh class A), moderate (Child-Pugh class B), and severe (Child-Pugh class C) hepatic impairment, respectively. In another study, the average AUC of everolimus in subjects with moderate hepatic impairment (Child-Pugh class B) was twice that found in subjects with normal hepatic function [see Dosage and Administration (2.10), Use in Specific Populations (8.6)].
Pediatric Patients: In patients with TSC-associated SEGA, the mean Cmin values normalized to mg/m2 dose in pediatric patients (<18 years of age) were lower than those observed in adults, suggesting that everolimus clearance adjusted to BSA was higher in pediatric patients as compared to adults.
Race or Ethnicity: Based on a cross-study comparison, Japanese patients had on average exposures that were higher than non-Japanese patients receiving the same dose. Oral clearance (CL/F) is on average 20% higher in black patients than in white patients.
Drug Interaction Studies
Effect of CYP3A4 and P-glycoprotein (P-gp) Inhibitors on Everolimus: Everolimus exposure increased when everolimus was coadministered with:
- ketoconazole (a P-gp and strong CYP3A4 inhibitor) - Cmax and AUC increased by 3.9- and 15-fold, respectively.
- erythromycin (a P-gp and moderate CYP3A4 inhibitor) - Cmax and AUC increased by 2- and 4.4-fold, respectively.
- verapamil (a P-gp and moderate CYP3A4 inhibitor) - Cmax and AUC increased by 2.3- and 3.5-fold, respectively.
Effect of CYP3A4 and P-gp Inducers on Everolimus: The coadministration of everolimus with rifampin, a P-gp and strong inducer of CYP3A4, decreased everolimus AUC by 63% and Cmax by 58% compared to everolimus alone [see Dosage and Administration (2.12)].
Effect of Everolimus on CYP3A4 Substrates: No clinically significant pharmacokinetic interactions were observed between everolimus and the HMG-CoA reductase inhibitors atorvastatin (a CYP3A4 substrate), pravastatin (a non- CYP3A4 substrate), and simvastatin (a CYP3A4 substrate).
The coadministration of an oral dose of midazolam (sensitive CYP3A4 substrate) with everolimus resulted in a 25% increase in midazolam Cmax and a 30% increase in midazolam AUC0-inf.
The coadministration of everolimus with exemestane increased exemestane Cmin
by 45% and C2h by 64%; however, the corresponding estradiol levels at steady
state (4 weeks) were not different between the 2 treatment arms. No increase
in adverse reactions related to exemestane was observed in patients with
hormone
receptor-positive, HER2-negative advanced breast cancer receiving the
combination.
The coadministration of everolimus with long-acting octreotide increased octreotide Cmin by approximately 50%.
Effect of Everolimus on Antiepileptic Drugs (AEDs): Everolimus increased pre- dose concentrations of the carbamazepine, clobazam, oxcarbazepine, and clobazam’s metabolite N-desmethylclobazam by about 10%. Everolimus had no impact on pre-dose concentrations of AEDs that are substrates of CYP3A4 (e.g., clonazepam and zonisamide) or other AEDs, including valproic acid, topiramate, phenobarbital, and phenytoin.
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
14.1 Hormone Receptor-Positive, HER2-Negative Breast Cancer
A randomized, double-blind, multicenter study (BOLERO-2, NCT00863655) of everolimus in combination with exemestane vs. placebo in combination with exemestane was conducted in 724 postmenopausal women with estrogen receptor- positive, HER2-negative advanced breast cancer with recurrence or progression following prior therapy with letrozole or anastrozole. Randomization was stratified by documented sensitivity to prior hormonal therapy (yes vs. no) and by the presence of visceral metastasis (yes vs. no). Sensitivity to prior hormonal therapy was defined as either (1) documented clinical benefit (complete response [CR], partial response [PR], stable disease ≥24 weeks) to at least one prior hormonal therapy in the advanced setting or (2) at least 24 months of adjuvant hormonal therapy prior to recurrence. Patients were permitted to have received 0-1 prior lines of chemotherapy for advanced disease. The major efficacy outcome measure was progression-free survival (PFS) evaluated by RECIST (Response Evaluation Criteria in Solid Tumors), based on investigator (local radiology) assessment. Other outcome measures included overall survival (OS) and objective response rate (ORR).
Patients were randomized 2:1 to everolimus 10 mg orally once daily in combination with exemestane 25 mg once daily (n=485) or to placebo in combination with exemestane 25 mg orally once daily (n=239). The two treatment groups were generally balanced with respect to baseline demographics and disease characteristics. Patients were not permitted to cross over to everolimus at the time of disease progression.
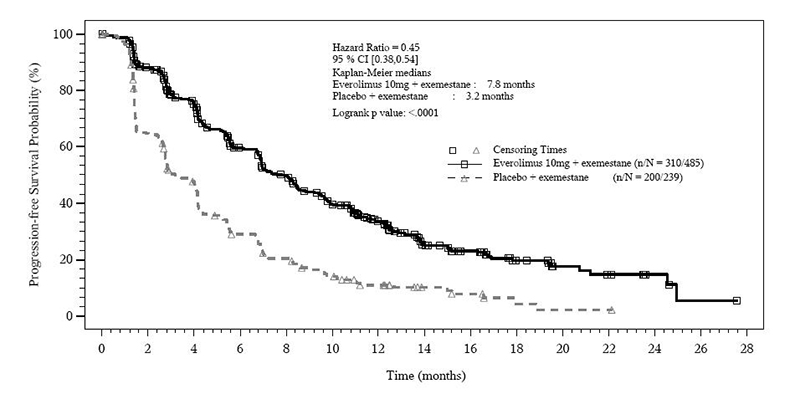
The trial demonstrated a statistically significant improvement in PFS by investigator assessment (Table 20 and Figure 1). The results of the PFS analysis based on independent central radiological assessment were consistent with the investigator assessment. PFS results were also consistent across the subgroups of age, race, presence and extent of visceral metastases, and sensitivity to prior hormonal therapy.
ORR was higher in the everolimus in combination with exemestane arm vs. the placebo in combination with exemestane arm (Table 20). There were 3 complete responses (0.6%) and 58 partial responses (12%) in the everolimus arm. There were no complete responses and 4 partial responses (1.7%) in the placebo in combination with exemestane arm.
After a median follow-up of 39.3 months, there was no statistically significant difference in OS between the everolimus in combination with exemestane arm and the placebo in combination with exemestane arm [HR 0.89 (95% CI: 0.73, 1.10)].
Table 20: Efficacy Results in Hormone-Receptor Positive, HER-2 Negative Breast Cancer in BOLERO-2|
Analysis |
Everolimus with Exemestane N=485 |
Placebo with Exemestane N=239 |
Hazard Ratio |
p-value |
|
Median progression-free survival (months, 95% CI) | ||||
|
Investigator radiological review |
7.8 |
3.2 |
0.45a |
<0.0001b |
|
(6.9, 8.5) |
(2.8, 4.1) |
(0.38, 0.54) | ||
|
Independent radiological review |
11.0 |
4.1 |
0.38a |
<0.0001b |
|
(9.7, 15.0) |
(2.9, 5.6) |
(0.3, 0.5) | ||
|
Best overall response (%, 95% CI) | ||||
|
Objective response rate (ORR)c |
12.6% |
1.7% |
n/ad | |
|
(9.8, 15.9) |
(0.5, 4.2) | |||
|
aHazard ratio is obtained from the stratified Cox proportional-hazards model by sensitivity to prior hormonal therapy and presence of visceral metastasis. | ||||
|
bp-value is obtained from the one-sided log-rank test stratified by sensitivity to prior hormonal therapy and presence of visceral metastasis. | ||||
|
cObjective response rate = proportion of patients with CR or PR. | ||||
|
dNot applicable. |
Figure 1: Kaplan-Meier Curves for Progression-Free Survival by Investigator Radiological Review in Hormone Receptor-Positive, HER-2 Negative Breast Cancer in BOLERO-2
14.2 Neuroendocrine Tumors (NET)
Pancreatic Neuroendocrine Tumors (PNET)
A randomized, double-blind, multicenter trial (RADIANT-3, NCT00510068) of everolimus in combination with best supportive care (BSC) compared to placebo in combination with BSC was conducted in patients with locally advanced or metastatic advanced PNET and disease progression within the prior 12 months. Patients were stratified by prior cytotoxic chemotherapy (yes vs. no) and WHO performance status (0 vs. 1 and 2). Treatment with somatostatin analogs was allowed as part of BSC. The major efficacy outcome was PFS evaluated by RECIST. After documented radiological progression, patients randomized to placebo could receive open-label everolimus. Other outcome measures included ORR, response duration, and OS.
Patients were randomized 1:1 to receive either everolimus 10 mg once daily (n=207) or placebo (n=203). Demographics were well balanced (median age 58 years, 55% male, 79% white). Of the 203 patients randomized to BSC, 172 patients (85%) received everolimus following documented radiologic progression.
The trial demonstrated a statistically significant improvement in PFS (Table 21 and Figure 2). PFS improvement was observed across all patient subgroups, irrespective of prior somatostatin analog use. The PFS results by investigator radiological review, central radiological review and adjudicated radiological review are shown below in Table 21.
Table 21: Progression-Free Survival Results in PNET in RADIANT-3|
Analysis |
N |
Everolimus N=207 |
Placebo N=203 | ||
|
410 |
Median progression-free survival (months) (95% CI) |
Hazard Ratio (95% CI) |
p-value | ||
|
Investigator radiological review |
11.0 |
4.6 |
0.35 |
<0.001 | |
|
(8.4, 13.9) |
(3.1, 5.4) |
(0.27, 0.45) | |||
|
Central radiological review |
13.7 |
5.7 |
0.38 |
<0.001 | |
|
(11.2, 18.8) |
(5.4, 8.3) |
(0.28, 0.51) | |||
|
Adjudicated radiological reviewa |
11.4 |
5.4 |
0.34 |
<0.001 | |
|
(10.8, 14.8) |
(4.3, 5.6) |
(0.26, 0.44) | |||
|
aIncludes adjudication for discrepant assessments between investigator radiological review and central radiological review. |
Figure 2: Kaplan-Meier Curves for Progression-Free Survival by Investigator Radiological Review in PNET in RADIANT-3
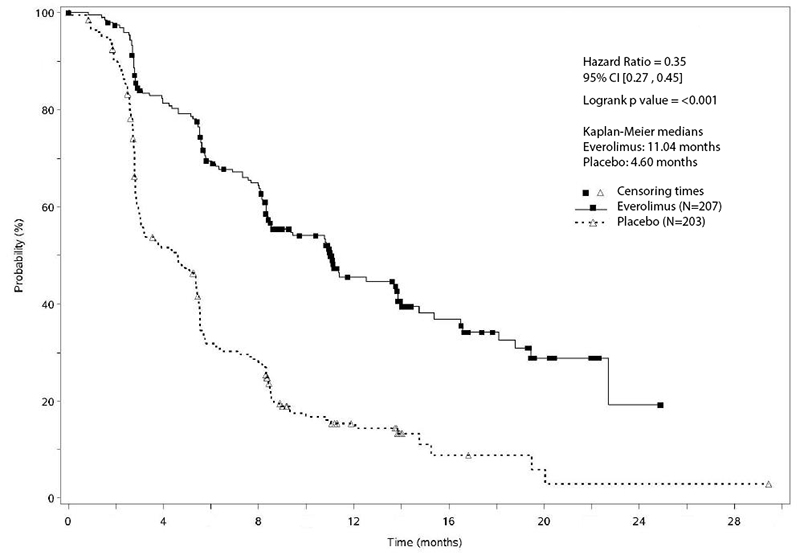
Investigator-determined response rate was 4.8% in the everolimus arm and there were no complete responses. Overall Survival (OS) was not statistically significantly different between arms [HR=0.94 (95% CI 0.73, 1.20); p=0.30].
NET of Gastrointestinal (GI) or Lung Origin
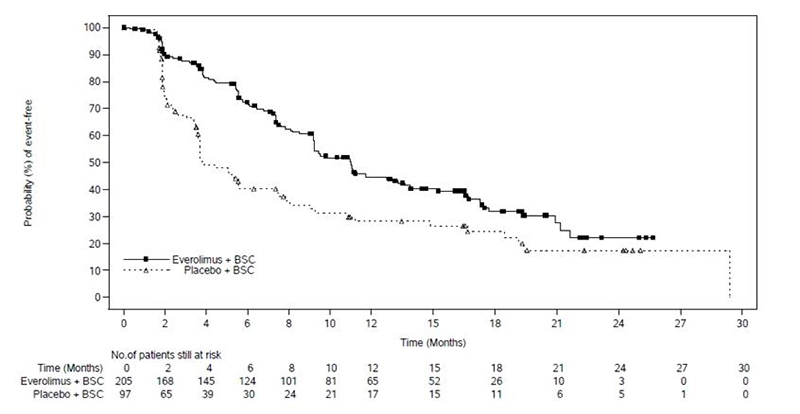
A randomized, double-blind, multicenter study (RADIANT-4, NCT01524783) of everolimus in combination with BSC compared to placebo in combination with BSC was conducted in patients with unresectable, locally advanced or metastatic, well differentiated, non-functional NET of GI (excluding pancreatic) or lung origin. The study required that patients had well-differentiated (low or intermediate grade) histology, no prior or current history of carcinoid symptoms, and evidence of disease progression within 6 months prior to randomization. Patients were randomized 2:1 to receive either everolimus 10 mg once daily or placebo, and stratified by prior somatostatin analog use (yes vs. no), tumor origin and WHO performance status (0 vs. 1). The major efficacy outcome measure was PFS based on independent radiological assessment evaluated by RECIST. Additional efficacy outcome measures were OS and ORR.
A total of 302 patients were randomized, 205 to the everolimus arm and 97 to the placebo arm. The median age was 63 years (22 to 86 years); 47% were male; 76% were white; 74% had WHO performance status of 0 and 26% had WHO performance status of 1. The most common primary sites of tumor were lung (30%), ileum (24%), and rectum (13%).
The study demonstrated a statistically significant improvement in PFS per independent radiological review (Table 22 and Figure 3). The final OS analysis did not show a statistically significant difference between those patients who received everolimus or placebo (HR = 0.90 [95% CI: 0.66, 1.24]).
Table 22: Progression-Free Survival in Neuroendocrine Tumors of Gastrointestinal or Lung Origin in RADIANT-4|
Everolimus N=205 |
Placebo N=97 | ||
|
Progression-Free Survival | |||
|
Number of Events |
113 (55%) |
65 (67%) | |
|
Progressive Disease |
104 (51%) |
60 (62%) | |
|
Death |
9 (4%) |
5 (5%) | |
|
Median PFS in months (95% CI) |
11.0 (9.2, 13.3) |
3.9 (3.6, 7.4) | |
|
Hazard Ratio (95% CI)a |
0.48 (0.35, 0.67) | ||
|
p-valueb |
<0.001 | ||
|
Overall Response Rate |
2% |
1% | |
|
aHazard ratio is obtained from the stratified Cox model. | |||
|
bp-value is obtained from the stratified log-rank test. |
Figure 3: Kaplan-Meier Curves for Progression-Free Survival in NET of GI or Lung Origin in RADIANT-4
Lack of Efficacy in Locally Advanced or Metastatic Functional Carcinoid Tumors
The safety and effectiveness of everolimus in patients with locally advanced or metastatic functional carcinoid tumors have not been demonstrated. In a randomized (1:1), double-blind, multicenter trial (RADIANT-2, NCT00412061) in 429 patients with carcinoid tumors, everolimus in combination with long-acting octreotide (Sandostatin LAR®) was compared to placebo in combination with long-acting octreotide. After documented radiological progression, patients on the placebo arm could receive everolimus; of those randomized to placebo, 67% received open-label everolimus in combination with long-acting octreotide. The study did not meet its major efficacy outcome measure of a statistically significant improvement in PFS and the final analysis of OS favored the placebo in combination with long-acting octreotide arm.
14.3 Renal Cell Carcinoma (RCC)
An international, multicenter, randomized, double-blind trial (RECORD-1, NCT00410124) comparing everolimus 10 mg once daily and placebo, both in conjunction with BSC, was conducted in patients with metastatic RCC whose disease had progressed despite prior treatment with sunitinib, sorafenib, or both sequentially. Prior therapy with bevacizumab, interleukin 2, or interferon-α was also permitted. Randomization was stratified according to prognostic score and prior anticancer therapy. The major efficacy outcome measure for the trial was PFS evaluated by RECIST, based on a blinded, independent, central radiologic review. After documented radiological progression, patients randomized to placebo could receive open-label everolimus. Other outcome measures included OS.
In total, 416 patients were randomized 2:1 to receive everolimus (n=277) or placebo (n=139). Demographics were well balanced between the arms (median age 61 years; 77% male, 88% white, 74% received prior sunitinib or sorafenib, and 26% received both sequentially).
Everolimus was superior to placebo for PFS (Table 23 and Figure 4). The treatment effect was similar across prognostic scores and prior sorafenib and/or sunitinib. Final OS results yield a hazard ratio of 0.90 (95% CI: 0.71, 1.14), with no statistically significant difference between the arms. Planned cross-over from placebo due to disease progression to open-label everolimus occurred in 80% of the 139 patients and may have confounded the OS benefit.
Table 23: Progression-Free Survival and Objective Response Rate by Central Radiologic Review in RCC in RECORD-1|
Everolimus N=277 |
Placebo N=139 |
Hazard Ratio (95% CI) |
p-value****a | |
|
Median Progression-free Survival |
4.9 months |
1.9 months |
0.33 |
<0.0001 |
|
(95% CI) |
(4.0, 5.5) |
(1.8, 1.9) |
(0.25, 0.43) | |
|
Objective Response Rate |
2% |
0% |
n/ab |
n/ab |
|
aLog-rank test stratified by prognostic score. | ||||
|
bNot applicable. |
Figure 4: Kaplan-Meier Curves for Progression-Free Survival in RCC in RECORD-1
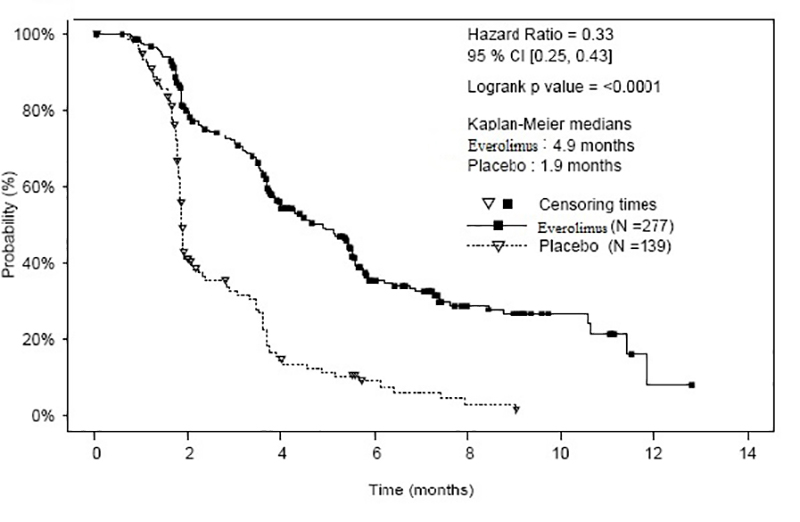
14.4 Tuberous Sclerosis Complex (TSC)-Associated Renal Angiomyolipoma
A randomized (2:1), double-blind, placebo-controlled trial (EXIST-2, NCT00790400) of everolimus was conducted in 118 patients with renal angiomyolipoma as a feature of TSC (n=113) or sporadic lymphangioleiomyomatosis (n=5). The key eligibility requirements for this trial were at least one angiomyolipoma of ≥3 cm in longest diameter on CT/MRI based on local radiology assessment, no immediate indication for surgery, and age ≥18 years. Patients received everolimus 10 mg or matching placebo orally once daily until disease progression or unacceptable toxicity. CT or MRI scans for disease assessment were obtained at baseline, 12, 24, and 48 weeks and annually thereafter. Clinical and photographic assessment of skin lesions were conducted at baseline and every 12 weeks thereafter until treatment discontinuation. The major efficacy outcome measure was angiomyolipoma response rate based on independent central radiology review, which was defined as a ≥50% reduction in angiomyolipoma volume, absence of new angiomyolipoma lesion ≥1 cm, absence of kidney volume increase ≥20%, and no angiomyolipoma related bleeding of ≥ Grade 2. Key supportive efficacy outcome measures were time to angiomyolipoma progression and skin lesion response rate. The primary analyses of efficacy outcome measures were limited to the blinded treatment period and conducted 6 months after the last patient was randomized. The comparative angiomyolipoma response rate analysis was stratified by use of enzyme-inducing antiepileptic drugs (EIAEDs) at randomization (yes vs. no).
Of the 118 patients enrolled, 79 were randomized to everolimus and 39 to placebo. The median age was 31 years (18 to 61 years), 34% were male, and 89% were white. At baseline, 17% of patients were receiving EIAEDs. On central radiology review at baseline, 92% of patients had at least 1 angiomyolipoma of ≥3 cm in longest diameter, 29% had angiomyolipomas ≥8 cm, 78% had bilateral angiomyolipomas, and 97% had skin lesions. The median values for the sum of all target renal angiomyolipoma lesions at baseline were 85 cm3 (9 to 1,612 cm3) and 120 cm3 (3 to 4,520 cm3) in the everolimus and placebo arms, respectively. Forty-six (39%) patients had prior renal embolization or nephrectomy. The median duration of follow-up was 8.3 months (0.7 to 24.8 months) at the time of the primary analysis.
The renal angiomyolipoma response rate was statistically significantly higher in everolimus-treated patients (Table 24). The median response duration was 5.3+ months (2.3+ to 19.6+ months).
There were 3 patients in the everolimus arm and 8 patients in the placebo arm with documented angiomyolipoma progression by central radiologic review (defined as a ≥25% increase from nadir in the sum of angiomyolipoma target lesion volumes to a value greater than baseline, appearance of a new angiomyolipoma ≥1 cm in longest diameter, an increase in renal volume ≥20% from nadir for either kidney and to a value greater than baseline, or Grade ≥2 angiomyolipoma-related bleeding). The time to angiomyolipoma progression was statistically significantly longer in the everolimus arm (HR 0.08 [95% CI: 0.02, 0.37]; p <0.0001).
Table 24: Angiomyolipoma Response Rate in TSC-Associated Renal Angiomyolipoma in EXIST-2|
Everolimus N=79 |
Placebo N=39 |
p-value | |
|
Primary analysis | |||
|
Angiomyolipoma response rate**a**– (%)**** |
41.8 |
0 |
<0.0001 |
|
95% CI |
(30.8, 53.4) |
(0.0, 9.0) | |
|
aPer independent central radiology review. |
Skin lesion response rates were assessed by local investigators for 77 patients in the everolimus arm and 37 patients in the placebo arm who presented with skin lesions at study entry. The skin lesion response rate was statistically significantly higher in the everolimus arm (26% vs. 0, p=0.0011); all skin lesion responses were partial responses, defined as visual improvement in 50% to 99% of all skin lesions durable for at least 8 weeks (Physician's Global Assessment of Clinical Condition).
Patients randomized to placebo were permitted to receive everolimus at the time of angiomyolipoma progression or after the time of the primary analysis. After the primary analysis, patients treated with everolimus underwent additional follow-up CT or MRI scans to assess tumor status until discontinuation of treatment or completion of 4 years of follow-up after the last patient was randomized. A total of 112 patients (79 randomized to everolimus and 33 randomized to placebo) received at least one dose of everolimus. The median duration of everolimus treatment was 3.9 years (0.5 months to 5.3 years) and the median duration of follow-up was 3.9 years (0.9 months to 5.4 years). During the follow-up period after the primary analysis, 32 patients (in addition to the 33 patients identified at the time of the primary analysis) had an angiomyolipoma response based upon independent central radiology review. Among the 65 responders out of 112 patients, the median time to angiomyolipoma response was 2.9 months (2.6 to 33.8 months). Fourteen percent of the 112 patients treated with everolimus had angiomyolipoma progression by the end of the follow-up period. No patient underwent a nephrectomy for angiomyolipoma progression and one patient underwent renal embolization while treated with everolimus.
14.5 Tuberous Sclerosis Complex (TSC)-Associated Subependymal Giant Cell
Astrocytoma (SEGA)
EXIST-1
A randomized (2:1), double-blind, placebo-controlled trial (EXIST-1, NCT00789828) of everolimus was conducted in 117 pediatric and adult patients with SEGA and TSC. Eligible patients had at least one SEGA lesion ≥1 cm in longest diameter on MRI based on local radiology assessment and one or more of the following: serial radiological evidence of SEGA growth, a new SEGA lesion ≥1 cm in longest diameter, or new or worsening hydrocephalus. Patients randomized to the treatment arm received everolimus at a starting dose of 4.5 mg/m2 daily, with subsequent dose adjustments as needed to achieve and maintain everolimus trough concentrations of 5 to 15 ng/mL as tolerated. Everolimus or matched placebo continued until disease progression or unacceptable toxicity. MRI scans for disease assessment were obtained at baseline, 12, 24, and 48 weeks, and annually thereafter.
The main efficacy outcome measure was SEGA response rate based on independent central radiology review. SEGA response was defined as a ≥50% reduction in the sum of SEGA volume relative to baseline, in the absence of unequivocal worsening of non-target SEGA lesions, a new SEGA lesion ≥1 cm, and new or worsening hydrocephalus. The primary analysis of SEGA response rate was limited to the blinded treatment period and conducted 6 months after the last patient was randomized. The analysis of SEGA response rate was stratified by use of enzyme-inducing antiepileptic drugs (EIAEDs) at randomization (yes vs. no).
Of the 117 patients enrolled, 78 were randomized to everolimus and 39 to placebo. The median age was 9.5 years (0.8 to 26 years); a total of 20 patients were <3 years, 54 patients were 3 to <12 years, 27 patients were 12 to <18 years, and 16 patients were ≥18 years; 57% were male, and 93% were white. At baseline, 18% of patients were receiving EIAEDs. Based on central radiology review at baseline, 98% of patients had at least one SEGA lesion ≥1.0 cm in longest diameter, 79% had bilateral SEGAs, 43% had ≥2 target SEGA lesions, 26% had growth in or into the inferior surface of the ventricle, 9% had evidence of growth beyond the subependymal tissue adjacent to the ventricle, and 7% had radiographic evidence of hydrocephalus. The median values for the sum of all target SEGA lesions at baseline were 1.63 cm3 (0.18 to 25.15 cm3) and 1.30 cm3 (0.32 to 9.75 cm3) in the everolimus and placebo arms, respectively. Eight (7%) patients had prior SEGA-related surgery. The median duration of follow-up was 8.4 months (4.6 to 17.2 months) at the time of primary analysis.
The SEGA response rate was statistically significantly higher in everolimus- treated patients (Table 25). At the time of the primary analysis, all SEGA responses were ongoing and the median duration of response was 5.3 months (2.1 to 8.4 months).
With a median follow-up of 8.4 months, SEGA progression was detected in 15.4% of the 39 patients randomized to receive placebo and none of the 78 patients randomized to receive everolimus. No patient in either treatment arm required surgical intervention.
Table 25: Subependymal Giant Cell Astrocytoma Response Rate in TSC- Associated SEGA in EXIST-1|
Everolimus N=78 |
Placebo N=39 |
p-value | |
|
Primary analysis | |||
|
** SEGA response ratea - (%)** |
35 |
0 |
<0.0001 |
|
95% CI |
24, 46 |
0, 9 | |
|
aPer independent central radiology review. |
Patients randomized to placebo were permitted to receive everolimus at the time of SEGA progression or after the primary analysis, whichever occurred first. After the primary analysis, patients treated with everolimus underwent additional follow-up MRI scans to assess tumor status until discontinuation of treatment or completion of 4 years of follow-up after the last patient was randomized. A total of 111 patients (78 patients randomized to everolimus and 33 patients randomized to placebo) received at least one dose of everolimus. Median duration of everolimus treatment and follow-up was 3.9 years (0.2 to 4.9 years).
By four years after the last patient was enrolled, 58% of the 111 patients treated with everolimus had a ≥50% reduction in SEGA volume relative to baseline, including 27 patients identified at the time of the primary analysis and 37 patients with a SEGA response after the primary analysis. The median time to SEGA response was 5.3 months (2.5 to 33.1 months). Twelve percent of the 111 patients treated with everolimus had documented disease progression by the end of the follow-up period and no patient required surgical intervention for SEGA during the study.
Study 2485
Study 2485 (NCT00411619) was an open-label, single-arm trial conducted to evaluate the antitumor activity of everolimus 3 mg/m2/orally once daily in patients with SEGA and TSC. Serial radiological evidence of SEGA growth was required for entry. Tumor assessments were performed every 6 months for 60 months after the last patient was enrolled or disease progression, whichever occurred earlier. The major efficacy outcome measure was the reduction in volume of the largest SEGA lesion with 6 months of treatment, as assessed via independent central radiology review. Progression was defined as an increase in volume of the largest SEGA lesion over baseline that was ≥25% over the nadir observed on study.
A total of 28 patients received everolimus for a median duration of 5.7 years (5 months to 6.9 years); 82% of the 28 patients remained on everolimus for at least 5 years. The median age was 11 years (3 to 34 years), 61% male, 86% white.
At the primary analysis, 32% of the 28 patients (95% CI: 16%, 52%) had an objective response at 6 months, defined as at least a 50% decrease in volume of the largest SEGA lesion. At the completion of the study, the median duration of durable response was 12 months (3 months to 6.3 years).
By 60 months after the last patient was enrolled, 11% of the 28 patients had documented disease progression. No patient developed a new SEGA lesion while on everolimus. Nine additional patients were identified as having a ≥50% volumetric reduction in their largest SEGA lesion between 1 to 4 years after initiating everolimus, including 3 patients who had surgical resection with subsequent regrowth prior to receiving everolimus.
REFERENCES SECTION
15 REFERENCES
1. OSHA Hazardous Drugs. OSHA. http://www.osha.gov/SLTC/hazardousdrugs/index.html.
SPL PATIENT PACKAGE INSERT SECTION
|
PATIENT INFORMATION | |
|
Read this Patient Information leaflet that comes with everolimus tablets before you start taking them and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or treatment. | |
|
What is the most important information I should know about everolimus tablets? Everolimus tablets can cause serious side effects, including: 1.You may develop lung or breathing problems. In some people lung or breathing problems may be severe and can lead to death. Tell your healthcare provider right away if you have any of these symptoms:
2.** You may be more likely to develop an infection**, such as pneumonia, or a bacterial, fungal or viral infection. Viral infections may include active hepatitis B in people who have had hepatitis B in the past (reactivation). In some people (including adults and children) these infections may be severe and can lead to death. You may need to be treated as soon as possible. Tell your healthcare provider right away if you have a temperature of 100.5˚F or above, chills, or do not feel well. Symptoms of hepatitis B or infection may include the following: | |
|
|
|
|
|
|
|
|
|
|
|
3.Severe allergic reactions. Call your healthcare provider or get medical help right away if you get signs and symptoms of a severe allergic reaction, including: rash, itching, hives, flushing, trouble breathing or swallowing, chest pain or dizziness. 4.** Possible increased risk for a type of allergic reaction called angioedema**, in people who take an Angiotensin-Converting Enzyme (ACE) inhibitor medicine during treatment with everolimus tablets. Talk with your healthcare provider before taking everolimus tablets if you are not sure if you take an ACE inhibitor medicine. Get medical help right away if you have trouble breathing or develop swelling of your tongue, mouth, or throat during treatment with everolimus tablets. 5.** Mouth ulcers and sores.** Mouth ulcers and sores are common during treatment with everolimus tablets but can also be severe. When you start treatment with everolimus tablets, your healthcare provider may tell you to also start a prescription mouthwash to reduce the likelihood of getting mouth ulcers or sores and to reduce their severity. Follow your healthcare provider’s instructions on how to use this prescription mouthwash. If you develop pain, discomfort, or open sores in your mouth, tell your healthcare provider. Your healthcare provider may tell you to restart this mouthwash or to use a special mouthwash or mouth gel that does not contain alcohol, peroxide, iodine, or thyme. 6.You may develop kidney failure. In some people this may be severe and can lead to death. Your healthcare provider should do tests to check your kidney function before and during your treatment with everolimus tablets. If you have any of the serious side effects listed above, you may need to stop taking everolimus tablets for a while or use a lower dose. Follow your healthcare provider’s instructions. | |
|
What are everolimus tablets? Everolimus tablets are a prescription medicine used to treat:
It is not known if everolimus tablets are safe and effective in children to treat:
| |
|
Do not take everolimus tablets if you have had a severe allergic reaction to everolimus. Talk to your healthcare provider before taking this medicine if you are allergic to:
Ask your healthcare provider if you do not know. | |
|
Before taking everolimus tablets, tell your healthcare provider about all of your medical conditions, including if you:
Females who are able to become pregnant:
Males with a female partner, you should use effective birth control during treatment and for 4 weeks after your last dose of everolimus tablets. Talk to your healthcare provider about birth control methods that may be right for you during this time. If you become pregnant or think you are pregnant, tell your healthcare provider right away.
Tell your healthcare provider about all of the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Everolimus tablets may affect the way other medicines work, and other medicines can affect how everolimus tablets work. Taking everolimus tablets with other medicines can cause serious side effects. Know the medicines you take. Keep a list of them and show it to your healthcare provider and pharmacist when you get a new medicine. Especially tell your healthcare provider if you take:
Ask your healthcare provider or pharmacist if you are not sure if your medicine is one of those taken for the conditions listed above. If you are taking any medicines for the conditions listed above, your healthcare provider might need to prescribe a different medicine or your dose of everolimus tablets may need to be changed. You should also tell your healthcare provider before you start taking any new medicine. | |
|
How should I take everolimus tablets?
| |
|
What should I avoid while taking everolimus tablets? | |
|
What are the possible side effects of everolimus tablets? Everolimus tablets can cause serious side effects, including: *See “What is the most important information I should know about everolimus tablets?” for more information. *Risk of wound healing problems. Wounds may not heal properly during everolimus tablets treatment. Tell your healthcare provider if you plan to have any surgery before starting or during treatment with everolimus tablets.
*Increased blood sugar and fat (cholesterol and triglyceride) levels in the blood. Your healthcare provider should do blood tests to check your fasting blood sugar, cholesterol, and triglyceride levels in the blood before you start and during treatment with everolimus tablets. *Decreased blood cell counts. Everolimus tablets can cause you to have decreased red blood cells, white blood cells, and platelets. Your healthcare provider should do blood tests to check your blood cell counts before you start and during treatment with everolimus tablets. *Worsening side effects from radiation treatment, that can sometimes be severe. Tell your healthcare provider if you have had or are planning to receive radiation therapy. The most common side effects of everolimus tablets in people with advanced hormone receptor-positive, HER2-negative breast cancer, advanced neuroendocrine tumors of the pancreas, stomach and intestine (gastrointestinal) or lung, and advanced kidney cancer include: | |
|
|
|
|
|
|
|
|
|
|
| |
|
**The most common side effects of everolimus tablets in people who have SEGA, renal angiomyolipoma, or certain types of seizures with TSC include **respiratory tract infections. Other side effects that may occur with everolimus tablets:
Tell your healthcare provider if you have any side effect that bothers you or does not go away. These are not all the possible side effects of everolimus tablets. For more information, ask your healthcare provider or pharmacist. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. | |
|
How should I store everolimus tablets?
Keep everolimus tablets and all medicines out of the reach of children. | |
|
General information about****the safe and effective use of ****everolimus tablets Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use everolimus tablets for a condition for which they were not prescribed. Do not give everolimus tablets to other people, even if they have the same problem you have. They may harm them. This leaflet summarizes the most important information about everolimus tablets. If you would like more information, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information written for healthcare professionals. | |
|
What are the ingredients in everolimus tablets? Manufactured In Croatia By:Pliva Hrvatska d.o.o., Zagreb, Croatia For more information, call Teva at 1-888-838-2872. |
This Patient Information has been approved by the U.S. Food and Drug Administration. Rev. E 8/2025
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosage Information
- Modify the dosage for patients with hepatic impairment or for patients taking drugs that inhibit or induce P-glycoprotein (P-gp) and CYP3A4 [see Dosage and Administration (2.10, 2.11, 2.12)].
2.2 Recommended Dosage for Hormone Receptor-Positive, HER2-Negative Breast
Cancer
The recommended dosage of everolimus tablets is 10 mg orally once daily until disease progression or unacceptable toxicity.
2.3 Recommended Dosage for Neuroendocrine Tumors (NET)
The recommended dosage of everolimus tablets is 10 mg orally once daily until disease progression or unacceptable toxicity.
2.4 Recommended Dosage for Renal Cell Carcinoma (RCC)
The recommended dosage of everolimus tablets is 10 mg orally once daily until disease progression or unacceptable toxicity.
2.5 Recommended Dosage for Tuberous Sclerosis Complex (TSC)-Associated
Renal Angiomyolipoma
The recommended dosage of everolimus tablets is 10 mg orally once daily until disease progression or unacceptable toxicity.
2.6 Recommended Dosage for Tuberous Sclerosis Complex (TSC)-Associated
Subependymal Giant Cell Astrocytoma (SEGA)
The recommended starting dosage of everolimus tablets is 4.5 mg/m2 orally once daily until disease progression or unacceptable toxicity [see Dosage and Administration (2.8)].
2.8 Therapeutic Drug Monitoring (TDM) and Dose Titration for Tuberous
Sclerosis Complex (TSC)-Associated Subependymal Giant Cell Astrocytoma (SEGA)
- Monitor everolimus whole blood trough concentrations at time points recommended in Table 1.
- Titrate the dose to attain trough concentrations of 5 ng/mL to 15 ng/mL.
- Adjust the dose using the following equation:
New dose* = current dose x (target concentration divided by current concentration)
*The maximum dose increment at any titration must not exceed 5 mg. Multiple dose titrations may be required to attain the target trough concentration.
- When possible, use the same assay and laboratory for TDM throughout treatment.
|
Event |
When to Assess Trough Concentrations After Event |
|
Initiation of everolimus tablets |
1 to 2 weeks |
|
Modification of everolimus tablets dose |
1 to 2 weeks |
|
Initiation or discontinuation of P-gp and moderate CYP3A4 inhibitor |
2 weeks |
|
Initiation or discontinuation of P-gp and strong CYP3A4 inducer |
2 weeks |
|
Change in hepatic function |
2 weeks |
|
Stable dose with changing body surface area (BSA) |
Every 3 to 6 months |
|
Stable dose with stable BSA |
Every 6 to 12 months |
|
Abbreviation: P-gp, P-glycoprotein. |
2.9 Dosage Modifications for Adverse Reactions
Table 2 summarizes recommendations for dosage modifications of everolimus tablets for the management of adverse reactions.
Table 2: Recommended Dosage Modifications for Everolimus Tablets for Adverse Reactions|
Adverse Reaction |
Severity |
Dosage Modification |
|
Non-infectious pneumonitis [see Warnings and Precautions (5.1)] |
Grade 2 |
Withhold until improvement to Grade 0 or 1. Resume at 50% of previous dose; change to every other day dosing if the reduced dose is lower than the lowest available strength. Permanently discontinue if toxicity does not resolve or improve to Grade 1 within 4 weeks. |
|
Grade 3 |
Withhold until improvement to Grade 0 or 1. Resume at 50% of previous dose; change to every other day dosing if the reduced dose is lower than the lowest available strength. If toxicity recurs at Grade 3, permanently discontinue. | |
|
Grade 4 |
Permanently discontinue. | |
|
Stomatitis [see Warnings and Precautions (5.5)] |
Grade 2 |
Withhold until improvement to Grade 0 or 1. Resume at same dose. If recurs at Grade 2, withhold until improvement to Grade 0 or 1. Resume at 50% of previous dose; change to every other day dosing if the reduced dose is lower than the lowest available strength. |
|
Grade 3 |
Withhold until improvement to Grade 0 or 1. Resume at 50% of previous dose; change to every other day dosing if the reduced dose is lower than the lowest available strength. | |
|
Grade 4 |
Permanently discontinue. | |
|
Metabolic events (e.g., hyperglycemia, dyslipidemia) [see Warnings and Precautions (5.9)] |
Grade 3 |
Withhold until improvement to Grade 0, 1, or 2. Resume at 50% of previous dose; change to every other day dosing if the reduced dose is lower than the lowest available strength. |
|
Grade 4 |
Permanently discontinue. | |
|
Other non-hematologic toxicities |
Grade 2 |
If toxicity becomes intolerable, withhold until improvement to Grade 0 or 1. Resume at same dose. If toxicity recurs at Grade 2, withhold until improvement to Grade 0 or 1. Resume at 50% of previous dose; change to every other day dosing if the reduced dose is lower than the lowest available strength. |
|
Grade 3 |
Withhold until improvement to Grade 0 or 1. Consider resuming at 50% of previous dose; change to every other day dosing if the reduced dose is lower than the lowest available strength. If recurs at Grade 3, permanently discontinue. | |
|
Grade 4 |
Permanently discontinue. | |
|
Thrombocytopenia [see Warnings and Precautions (5.10)] |
Grade 2 |
Withhold until improvement to Grade 0 or 1. Resume at same dose. |
|
Grade 3 OR Grade 4 |
Withhold until improvement to Grade 0 or 1. Resume at 50% of previous dose; change to every other day dosing if the reduced dose is lower than the lowest available strength. | |
|
Neutropenia [see Warnings and Precautions (5.10)] |
Grade 3 |
Withhold until improvement to Grade 0, 1, or 2. Resume at same dose. |
|
Grade 4 |
Withhold until improvement to Grade 0, 1, or 2. Resume at 50% of previous dose; change to every other day dosing if the reduced dose is lower than the lowest available strength. | |
|
Febrile neutropenia [see Warnings and Precautions (5.10)] |
Grade 3 |
Withhold until improvement to Grade 0, 1, or 2, and no fever. Resume at 50% of previous dose; change to every other day dosing if the reduced dose is lower than the lowest available strength. |
|
Grade 4 |
Permanently discontinue. |
2.10 Dosage Modifications for Hepatic Impairment
The recommended dosages of everolimus tablets for patients with hepatic impairment are described in Table 3 [see Use in Specific Populations (8.6)]:
Table 3: Recommended Dosage Modifications for Patients with Hepatic Impairment|
Indication |
Dose Modification for Everolimus Tablets |
|
Breast Cancer, NET, RCC, and TSC-Associated Renal Angiomyolipoma |
|
|
TSC-Associated SEGA |
|
|
Abbreviations: NET, Neuroendocrine Tumors; RCC, Renal Cell Carcinoma; SEGA, Subependymal Giant Cell Astrocytoma; TSC, Tuberous Sclerosis Complex. |
2.11 Dosage Modifications for P-gp and CYP3A4 Inhibitors
-
Avoid the concomitant use of P-gp and strong CYP3A4 inhibitors [see Drug Interactions (7.1)].
-
Avoid ingesting grapefruit and grapefruit juice.
-
Reduce the dose for patients taking everolimus tablets with a P-gp and moderate CYP3A4 inhibitor as recommended in Table 4 [see Drug Interactions (7.1), Clinical Pharmacology (12.3)].
|
Indication |
Dose Modification for Everolimus Tablets |
|
Breast Cancer, NET, RCC, and TSC-Associated Renal Angiomyolipoma |
|
|
TSC-Associated SEGA |
|
2.12 Dosage Modifications for P-gp and CYP3A4 Inducers
- Avoid concomitant use of St. John’s Wort (Hypericum perforatum).
- Increase the dose for patients taking everolimus tablets with a P-gp and strong CYP3A4 inducer as recommended in Table 5 [see Drug Interactions (7.1), Clinical Pharmacology (12.3)].
|
Indication |
Dose Modification for Everolimus Tablets |
|
Breast Cancer, NET, RCC, and |
|
|
TSC-Associated SEGA |
|
2.13 Administration and Preparation
- Administer everolimus tablets at the same time each day.
- Administer everolimus tablets consistently either with or without food [see Clinical Pharmacology (12.3)].
- If a dose of everolimus tablets is missed, it can be administered up to 6 hours after the time it is normally administered. After more than 6 hours, the dose should be skipped for that day. The next day, everolimus tablets should be administered at its usual time. Double doses should not be administered to make up for the dose that was missed.
- Everolimus tablets should be swallowed whole with a glass of water. Do not break or crush tablets.
Modify the dose for patients with hepatic impairment or for patients taking drugs that inhibit or induce P-glycoprotein (P-gp) and CYP3A4. (2.1)
Breast Cancer:
- 10 mg orally once daily. (2.2)
NET:
- 10 mg orally once daily. (2.3)
RCC:
- 10 mg orally once daily. (2.4)
TSC-Associated Renal Angiomyolipoma:
- 10 mg orally once daily. (2.5)
TSC-Associated SEGA:
- 4.5 mg/m2 orally once daily; adjust dose to attain trough concentrations of 5 to 15 ng/mL. (2.6, 2.8)
DOSAGE FORMS & STRENGTHS SECTION
3 DOSAGE FORMS AND STRENGTHS
2.5 mg tablet – White to slightly yellow, elongated tablets with a bevelled edge and no score, debossed with “TEVA” on one side and “7766” on the other side.
5 mg tablet – White to slightly yellow, elongated tablets with a bevelled edge and no score, debossed with “TEVA” on one side and “7767” on the other side.
7.5 mg tablet – White to slightly yellow, elongated tablets with a bevelled edge and no score, debossed with “TEVA” on one side and “7768” on the other side.
10 mg tablet – White to slightly yellow, elongated tablets with a bevelled edge and no score, debossed with “TEVA” on one side and “7769” on the other side.
- Everolimus tablets: 2.5 mg, 5 mg, 7.5 mg, and 10 mg tablets (3)
USE IN SPECIFIC POPULATIONS SECTION
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on animal studies and the mechanism of action [see Clinical Pharmacology (12.1)], everolimus can cause fetal harm when administered to a pregnant woman. There are limited case reports of everolimus use in pregnant women; however, these reports are not sufficient to inform about risks of birth defects or miscarriage. In animal studies, everolimus caused embryo-fetal toxicities in rats when administered during the period of organogenesis at maternal exposures that were lower than human exposures at the recommended dose of everolimus 10 mg orally once daily (see Data). Advise pregnant women of the potential risk to the fetus.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage is 2% to 4% and 15% to 20% of clinically recognized pregnancies, respectively.
Data
Animal Data
In animal reproductive studies, oral administration of everolimus to female
rats before mating and through organogenesis induced embryo-fetal toxicities,
including increased resorption, pre-implantation and
post-implantation loss, decreased numbers of live fetuses, malformation (e.g.,
sternal cleft), and retarded skeletal development. These effects occurred in
the absence of maternal toxicities. Embryo-fetal toxicities in rats occurred
at doses ≥0.1 mg/kg (0.6 mg/m2) with resulting exposures of approximately 4%
of the human exposure at the recommended dose of everolimus 10 mg orally once
daily based on area under the curve (AUC). In rabbits, embryo-toxicity evident
as an increase in resorptions occurred at an oral dose of 0.8 mg/kg (9.6
mg/m2), approximately 1.6 times the recommended dose of everolimus 10 mg
orally once daily or the median dose administered to patients with tuberous
sclerosis complex (TSC)-associated subependymal giant cell astrocytoma (SEGA).
The effect in rabbits occurred in the presence of maternal toxicities.
In a pre- and post-natal development study in rats, animals were dosed from implantation through lactation. At the dose of 0.1 mg/kg (0.6 mg/m2), there were no adverse effects on delivery and lactation or signs of maternal toxicity; however, there were reductions in body weight (up to 9% reduction from the control) and in survival of offspring (~5% died or missing). There were no drug-related effects on the developmental parameters (morphological development, motor activity, learning, or fertility assessment) in the offspring.
8.2 Lactation
Risk Summary
There are no data on the presence of everolimus or its metabolites in human milk, the effects of everolimus on the breastfed infant or on milk production. Everolimus and its metabolites passed into the milk of lactating rats at a concentration 3.5 times higher than in maternal serum. Because of the potential for serious adverse reactions in breastfed infants from everolimus, advise women not to breastfeed during treatment with everolimus and for 2 weeks after the last dose.
8.3 Females and Males of Reproductive Potential
Pregnancy Testing
Verify the pregnancy status of females of reproductive potential prior to starting everolimus [see Use in Specific Populations (8.1)].
Contraception
Everolimus can cause fetal harm when administered to pregnant women [see Use in Specific Populations (8.1)].
Females: Advise female patients of reproductive potential to use effective contraception during treatment with everolimus and for 8 weeks after the last dose.
Males: Advise male patients with female partners of reproductive potential to use effective contraception during treatment with everolimus and for 4 weeks after the last dose.
Infertility
Females: Menstrual irregularities, secondary amenorrhea, and increases in luteinizing hormone (LH) and follicle stimulating hormone (FSH) occurred in female patients taking everolimus. Based on these findings, everolimus may impair fertility in female patients [see Adverse Reactions (6.1), Nonclinical Toxicology (13.1)].
Males: Cases of reversible azoospermia have been reported in male patients taking everolimus. In male rats, sperm motility, sperm count, plasma testosterone levels and fertility were diminished at AUC similar to those of the clinical dose of everolimus 10 mg orally once daily. Based on these findings, everolimus may impair fertility in male patients [see Nonclinical Toxicology (13.1)].
8.4 Pediatric Use
TSC-Associated SEGA
The safety and effectiveness of everolimus have been established in pediatric patients age 1 year and older with TSC-associated SEGA that requires therapeutic intervention but cannot be curatively resected. Use of everolimus for this indication is supported by evidence from a randomized, double-blind, placebo-controlled trial in adult and pediatric patients (EXIST-1); an open- label, single-arm trial in adult and pediatric patients (Study 2485); and additional pharmacokinetic data in pediatric patients [see Adverse Reactions (6.1), Clinical Pharmacology (12.3), Clinical Studies (14.5)]. The safety and effectiveness of everolimus have not been established in pediatric patients less than 1 year of age with TSC-associated SEGA.
In EXIST-1, the incidence of infections and serious infections were reported
at a higher frequency in patients
<6 years of age. Ninety-six percent of 23 everolimus-treated patients <6 years
had at least one infection compared to 67% of 55 everolimus-treated patients
≥6 years. Thirty-five percent of 23 everolimus-treated patients <6 years of
age had at least 1 serious infection compared to 7% of 55 everolimus-treated
patients ≥6 years.
Although a conclusive determination cannot be made due to the limited number of patients and lack of a comparator arm in the open label follow-up periods of EXIST-1 and Study 2485, everolimus did not appear to adversely impact growth and pubertal development in the 115 pediatric patients treated with everolimus for a median duration of 4.1 years.
Other Indications
The safety and effectiveness of everolimus in pediatric patients have not been established in:
- Hormone receptor-positive, HER2-negative breast cancer
- Neuroendocrine tumors (NET)
- Renal cell carcinoma (RCC)
- TSC-associated renal angiomyolipoma
8.5 Geriatric Use
In BOLERO-2, 40% of patients with breast cancer treated with everolimus were ≥65 years of age, while 15% were ≥75 years of age. No overall differences in effectiveness were observed between elderly and younger patients. The incidence of deaths due to any cause within 28 days of the last everolimus dose was 6% in patients ≥65 years of age compared to 2% in patients <65 years of age. Adverse reactions leading to permanent treatment discontinuation occurred in 33% of patients ≥65 years of age compared to 17% in patients <65 years of age.
In RECORD-1, 41% of patients with renal cell carcinoma treated with everolimus were ≥65 years of age, while 7% were ≥75 years of age. In RADIANT-3, 30% of patients with PNET treated with everolimus were ≥65 years of age, while 7% were ≥75 years of age. No overall differences in safety or effectiveness were observed between elderly and younger patients.
8.6 Hepatic Impairment
Everolimus exposure may increase in patients with hepatic impairment [see Clinical Pharmacology (12.3)].
For patients with breast cancer, NET, RCC, and TSC-associated renal angiomyolipoma who have hepatic impairment, reduce the everolimus dose as recommended [see Dosage and Administration (2.10)].
For patients with TSC-associated SEGA who have severe hepatic impairment (Child-Pugh class C), reduce the starting dose of everolimus as recommended and adjust the dose based on everolimus trough concentrations [see Dosage and Administration (2.8, 2.10)].
- For breast cancer, NET, RCC, or TSC-associated renal angiomyolipoma patients with hepatic impairment, reduce the dose. (2.10, 8.6)
- For patients with TSC-associated SEGA and severe hepatic impairment, reduce the starting dose and adjust dose to attain target trough concentrations. (2.8, 2.10, 8.6)
DESCRIPTION SECTION
11 DESCRIPTION
Everolimus tablets are a kinase inhibitor.
The chemical name of everolimus, USP is (1R,9S,12S,15R,16E,18R,19R,21R,23S,24E,26E,28E,30S,32S,35R)-1,18-dihydroxy-12-{(1R)-2-[(1S,3R,4R)-4-(2-hydroxyethoxy)-3-methoxycyclohexyl]-1-methylethyl}-19,30-dimethoxy-15,17,21,23,29,35-hexamethyl-11,36-dioxa-4-aza- tricyclo[30.3.1.04,9]hexatriaconta-16,24,26,28-tetraene-2,3,10,14,20-pentaone. The molecular formula is C53H83NO14 and the molecular weight is 958.2. The structural formula is:
Everolimus tablets for oral administration contain 2.5 mg, 5 mg, 7.5 mg, or 10 mg of everolimus, USP and the following inactive ingredients: butylated hydroxytoluene, crospovidone, hypromellose 2910, lactose anhydrous, lactose monohydrate, and magnesium stearate.
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Administration of everolimus for up to 2 years did not indicate oncogenic potential in mice and rats up to the highest doses tested (0.9 mg/kg) corresponding, respectively to 3.9 and 0.2 times the estimated human exposure based on AUC at the recommended dose of everolimus 10 mg orally once daily.
Everolimus was not genotoxic in a battery of in vitro assays (Ames mutation test in Salmonella, mutation test in L5178Y mouse lymphoma cells, and chromosome aberration assay in V79 Chinese hamster cells). Everolimus was not genotoxic in an in vivo mouse bone marrow micronucleus test at doses up to 500 mg/kg/day (1,500 mg/m2/day, approximately 255-fold the recommended dose of everolimus 10 mg orally once daily, and approximately 200-fold the median dose administered to patients with TSC-associated SEGA, based on the BSA), administered as 2 doses, 24 hours apart.
Based on non-clinical findings, everolimus may impair male fertility. In a 13-week male fertility study in rats, testicular morphology was affected at doses of 0.5 mg/kg and above. Sperm motility, sperm count, and plasma testosterone levels were diminished in rats treated with 5 mg/kg. The exposures at these doses (52 ng•hr/mL and 414 ng•hr/mL, respectively) were within the range of human exposure at the recommended dose of everolimus 10 mg orally once daily (560 ng•hr/mL) and resulted in infertility in the rats at 5 mg/kg. Effects on male fertility occurred at AUC0-24h values 10% to 81% lower than human exposure at the recommended dose of everolimus 10 mg orally once daily. After a 10 to 13 week non-treatment period, the fertility index increased from zero (infertility) to 60%.
Oral doses of everolimus in female rats at doses ≥0.1 mg/kg (approximately 4% the human exposure based on AUC at the recommended dose of everolimus 10 mg orally once daily) resulted in increased incidence of pre-implantation loss, suggesting that the drug may reduce female fertility.
13.2 Animal Toxicology and/or Pharmacology
In juvenile rat toxicity studies, dose-related delayed attainment of developmental landmarks, including delayed eye-opening, delayed reproductive development in males and females and increased latency time during the learning and memory phases were observed at doses as low as 0.15 mg/kg/day.
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Non-infectious Pneumonitis
Advise patients of the risk of developing non-infectious pneumonitis and to immediately report any new or worsening respiratory symptoms to their healthcare provider [see Warnings and Precautions (5.1)].
Infections
Advise patients that they are more susceptible to infections and that they should immediately report any signs or symptoms of infections to their healthcare provider [see Warnings and Precautions (5.2)].
Hypersensitivity Reactions
Advise patients of the risk of clinically significant hypersensitivity reactions and to promptly contact their healthcare provider or seek emergency care for signs of hypersensitivity reaction, including rash, itching, hives, difficulty breathing or swallowing, flushing, chest pain, or dizziness [see Contraindications (4), Warnings and Precautions (5.3)].
Angioedema with Concomitant Use of ACE Inhibitors
Advise patients to avoid ACE inhibitors and to promptly contact their healthcare provider or seek emergency care for signs or symptoms of angioedema [see Warnings and Precautions (5.4)].
Stomatitis
Advise patients of the risk of stomatitis and to use alcohol-free mouthwashes during treatment [see Warnings and Precautions (5.5)].
Renal Impairment
Advise patients of the risk of developing kidney failure and the need to monitor their kidney function periodically during treatment [see Warnings and Precautions (5.6)].
Risk of Impaired Wound Healing
Advise patients that everolimus tablets may impair wound healing. Advise patients to inform their healthcare provider of any planned surgical procedure [see Warnings and Precautions (5.7)].
Geriatric Patients
Inform patients that in a study conducted in patients with breast cancer, the incidence of deaths and adverse reactions leading to permanent discontinuation was higher in patients ≥65 years compared to patients <65 years [see Warnings and Precautions (5.8), Use in Specific Populations (8.5)].
Metabolic Disorders
Advise patients of the risk of metabolic disorders and the need to monitor glucose and lipids periodically during therapy [see Warnings and Precautions (5.9)].
Myelosuppression
Advise patients of the risk of myelosuppression and the need to monitor CBCs periodically during therapy [see Warnings and Precautions (5.10)].
Risk of Infection or Reduced Immune Response with Vaccination
Advise patients to avoid the use of live vaccines and close contact with those who have received live vaccines [see Warnings and Precautions (5.11)].
Embryo-Fetal Toxicity
Advise females of reproductive potential of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment and for 8 weeks after the last dose. Advise patients to inform their healthcare provider of a known or suspected pregnancy. Advise males with female partners of reproductive potential to use effective contraception during treatment and for 4 weeks after the last dose [see Warnings and Precautions (5.13), Use in Specific Populations (8.1, 8.3)].
Radiation Sensitization and Radiation Recall
Radiation sensitization and recall can occur in patients treated with radiation prior to, during, or subsequent to everolimus treatment. Advise patients to inform their healthcare provider if they have had or are planning to receive radiation therapy [see Warnings and Precautions (5.12)].
Lactation
Advise women not to breastfeed during treatment with everolimus and for 2 weeks after the last dose [see Use in Specific Populations (8.2)].
Infertility
Advise males and females of reproductive potential of the potential risk for impaired fertility [see Use in Specific Populations (8.3)].
Brands listed are the trademarks of their respective owners.
Manufactured In Croatia By:
Pliva Hrvatska d.o.o.
****Zagreb, Croatia
Manufactured For:
Teva Pharmaceuticals
****Parsippany, NJ 07054
Rev. E 8/2025
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
Everolimus Tablets
2.5 mg tablets
White to slightly yellow, elongated tablets with a bevelled edge and no score
debossed with “TEVA” on one side and “7766” on the other side; available in:
NDC 0093-7766-24 – Carton containing 4 blister cards of 7 tablets each (28 tablets total).
5 mg tablets
White to slightly yellow, elongated tablets with a bevelled edge and no score debossed with “TEVA” on one side and “7767” on the other side; available in:
NDC 0093-7767-24 – Carton containing 4 blister cards of 7 tablets each (28 tablets total).
7.5 mg tablets
White to slightly yellow, elongated tablets with a bevelled edge and no score
debossed with “TEVA” on one side and “7768” on the other side; available in:
NDC 0093-7768-24 – Carton containing 4 blister cards of 7 tablets each (28 tablets total).
10 mg tablets
White to slightly yellow, elongated tablets with a bevelled edge and no score
debossed with “TEVA” on one side and “7769” on the other side; available in:
NDC 0093-7769-24 – Carton containing 4 blister cards of 7 tablets each (28 tablets total).
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
Store in the original container, protect from light and moisture.
Keep this and all drugs out of the reach of children.
Follow special handling and disposal procedures for anti-cancer pharmaceuticals.1
