Registrants1
Companies and organizations registered with the FDA for this drug approval, including their contact information and regulatory details.
527695995
Manufacturing Establishments1
FDA-registered manufacturing facilities and establishments involved in the production, packaging, or distribution of this drug product.
Northstar Rx LLC
Novast Laboratories, Ltd.
527695995
Products1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
SETLAKIN
Product Details
Drug Labeling Information
Complete FDA-approved labeling information including indications, dosage, warnings, contraindications, and other essential prescribing details.
ADVERSE REACTIONS SECTION
Highlight: The most common adverse reactions (≥2%) reported during clinical trials were headache, menorrhagia, nausea, dysmenorrhea, acne, migraine, breast tenderness, weight increased, and depression. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Northstar Rx LLC at 1-800-206-7821 or FDA at 1-800-FDA-1088 orwww.fda.gov/medwatch.
6 ADVERSE REACTIONS
The following serious adverse reactions with the use of COCs are discussed elsewhere in the labeling:
- Serious cardiovascular events and stroke [see Boxed Warning and Warnings and Precautions (5.1)]
- Vascular events [see Warnings and Precautions (5.1)]
- Liver disease [see Warnings and Precautions (5.2)]
6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to the rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The clinical trial that evaluated the safety and efficacy of levonorgestrel and ethinyl estradiol 0.15 mg/0.03mg (91-day cycle) was a 12-month, randomized, multicenter, open-label study, which enrolled women aged 18 to 40, of whom 456 took at least one dose of levonorgestrel and ethinyl estradiol 0.15 mg/0.03mg (345.14 woman-years of exposure) [see Clinical Studies (14)].
Adverse Reactions Leading to Study Discontinuation: 14.9% of the women discontinued from the clinical trial due to an adverse reaction; the most common adverse reactions (≥ 1% of women) leading to discontinuation in the levonorgestrel and ethinyl estradiol 0.15 mg/0.03mg (91-day cycle) group were menorrhagia (5.7%), mood swings (1.9%), weight/appetite increase (1.5%), and acne (1.3%).
Common Adverse Reactions (≥ 2% of women): headache (20.6%), menorrhagia (11.6%), nausea (7.5%), dysmenorrhea (5.7%), acne (4.6%), migraine (4.4%), breast tenderness (3.5%), weight increased (3.1%), and depression (2.1%).
Serious Adverse Reactions: pulmonary embolus, cholecystitis.
6.2 Postmarketing Experience
Five studies that compared breast cancer risk between ever-users (current or past use) of COCs and never-users of COCs reported no association between ever use of COCs and breast cancer risk, with effect estimates ranging from 0.90 - 1.12 (Figure 2).
Three studies compared breast cancer risk between current or recent COC users (<6 months since last use) and never users of COCs (Figure 2). One of these studies reported no association between breast cancer risk and COC use. The other two studies found an increased relative risk of 1.19 - 1.33 with current or recent use. Both of these studies found an increased risk of breast cancer with current use of longer duration, with relative risks ranging from 1.03 with less than one year of COC use to approximately 1.4 with more than 8-10 years of COC use.
Figure 2: Relevant Studies of Risk of Breast Cancer with Combined Oral Contraceptives
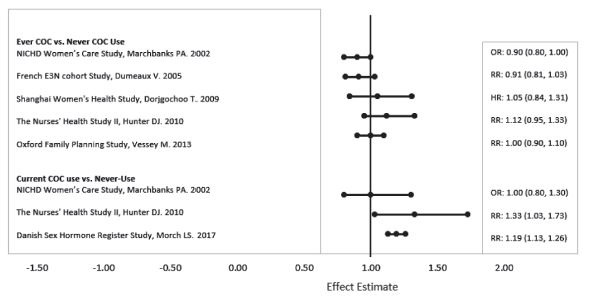
RR = relative risk; OR = odds ratio; HR = hazard ratio. “ever COC” are females with current or past COC use; “never COC use” are females that never used COCs.
The following adverse reactions have been identified during post-approval use of levonorgestrel and ethinyl estradiol 0.15 mg/0.03mg (91-day cycle). Because these reactions are reported voluntarily from a population of uncertain size, it is not possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Gastrointestinal disorders: abdominal distension, vomiting
General disorders and administration site conditions: chest pain, fatigue, malaise, edema peripheral, pain
Immune system disorder: hypersensitivity reactions, including itching, rash, and angioedema
Investigations: blood pressure increased
Musculoskeletal and connective tissue disorders: muscle spasms, pain in extremity
Nervous system disorders: dizziness, loss of consciousness
Psychiatric disorders: insomnia
Reproductive and breast disorders: dysmenorrhea
Skin and subcutaneous tissue disorders: alopecia
Vascular disorders: thrombosis, pulmonary embolism, pulmonary thrombosis
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
See Warnings and Precautions (5.2, 5.11).
