Olopatadine hydrochloride
Drug Facts
616da51c-70f4-3f87-320f-4685ad1520fa
HUMAN OTC DRUG LABEL
Sep 19, 2025
Apotex Corp.
DUNS: 845263701
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Olopatadine hydrochloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
Drug Labeling Information
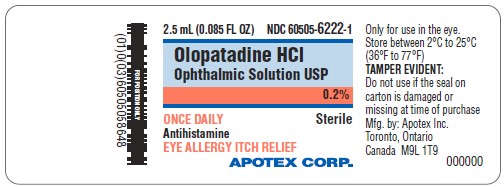
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Principal Display Panel
ONCE DAILY RELIEF
Olopatadine HCl Ophthalmic Solution, USP 0.2%
Antihistamine
Eye Allergy Itch Relief
NDC 60505-6222-2
INDICATIONS & USAGE SECTION
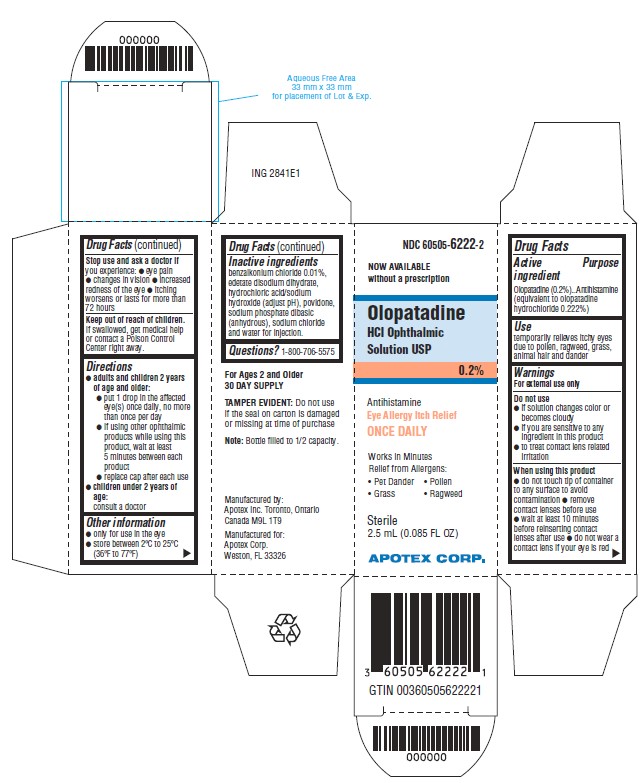
Uses
Temporarily relieves itchy eyes due to pollen, ragweed, grass, animal hair and dander.
SPL UNCLASSIFIED SECTION
Other information
- Only for use in the eye
- Store between 2ºC to 25ºC (36ºF to 77ºF)
OTC - ACTIVE INGREDIENT SECTION
Active ingredient
Olopatadine (0.2%) (equivalent to olopatadine hydrochloride 0.222%)
OTC - PURPOSE SECTION
Purpose
Antihistamine
WARNINGS SECTION
Warnings
For external use only
Do not use
- If solution changes color or becomes cloudy
- If you are sensitive to any ingredient in this product
- To treat contact lens related irritation
When using this product
- Do not touch tip of container to any surface to avoid contamination
- Remove contact lenses before use
- Wait at least 10 minutes before reinserting contact lenses after use
- Do not wear a contact lens if your eye is red
Stop use and ask a doctor if****you experience:
- Eye pain
- Changes in vision
- Increased redness of the eye
- Itching worsens or lasts for more than 72 hours
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
DOSAGE & ADMINISTRATION SECTION
Directions
*adults and children 2 years and older: * put 1 drop in the affected eye(s) once daily, no more than once per day * if using other ophthalmic products while using this product, wait at least 5 minutes between each product * replace cap after each use *children under 2 years of age:
Consult a doctor
INACTIVE INGREDIENT SECTION
Inactive ingredients
benzalkonium chloride 0.01%, dibasic sodium phosphate, edetate disodium dihydrate, hydrochloric acid and/or sodium hydroxide (to adjust pH), povidone, sodium chloride and water for injection
OTC - QUESTIONS SECTION
Questions?
1-800-706-5575
