Xylocaine
Xylocaine® (lidocaine HCl Injection, USP) Xylocaine® (lidocaine HCl and epinephrine Injection, USP)
ba082c2f-64f4-419d-9c88-74f203316e17
HUMAN PRESCRIPTION DRUG LABEL
Aug 25, 2021
Fresenius Kabi USA, LLC
DUNS: 608775388
Products 10
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
LIDOCAINE HYDROCHLORIDE,EPINEPHRINE BITARTRATE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
LIDOCAINE HYDROCHLORIDE,EPINEPHRINE BITARTRATE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (8)
LIDOCAINE HYDROCHLORIDE,EPINEPHRINE BITARTRATE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (8)
LIDOCAINE HYDROCHLORIDE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
LIDOCAINE HYDROCHLORIDE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (4)
LIDOCAINE HYDROCHLORIDE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (4)
LIDOCAINE HYDROCHLORIDE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (5)
LIDOCAINE HYDROCHLORIDE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (4)
LIDOCAINE HYDROCHLORIDE,EPINEPHRINE BITARTRATE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
LIDOCAINE HYDROCHLORIDE,EPINEPHRINE BITARTRATE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (8)
Drug Labeling Information
DESCRIPTION SECTION
DESCRIPTION
Xylocaine (lidocaine HCl) Injections are sterile, nonpyrogenic, aqueous solutions that contain a local anesthetic agent with or without epinephrine and are administered parenterally by injection. SeeINDICATIONS AND USAGE section for specific uses.
Xylocaine solutions contain lidocaine HCl, which is chemically designated as acetamide, 2-(diethylamino)-N-(2,6-dimethylphenyl)-, monohydrochloride and has the molecular wt. 270.8. Lidocaine HCl (C14H22N2O • HCl) has the following structural formula:
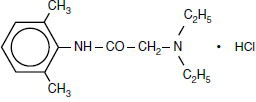
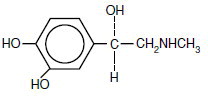
Epinephrine is (-) -3, 4-Dihydroxy-α-[(methylamino) methyl] benzyl alcohol and has the molecular wt. 183.21. Epinephrine (C9H13NO3) has the following structural formula:
Dosage forms listed as Xylocaine-MPF indicate single dose solutions that are MethylParabenFree (MPF).
Xylocaine MPF is a sterile, nonpyrogenic, isotonic solution containing sodium chloride. Xylocaine in multiple dose vials: Each mL also contains 1 mg methylparaben as antiseptic preservative. The pH of these solutions is adjusted to approximately 6.5 (5.0 to 7.0) with sodium hydroxide and/or hydrochloric acid.
Xylocaine MPF with Epinephrine is a sterile, nonpyrogenic, isotonic solution containing sodium chloride. Each mL contains lidocaine hydrochloride and epinephrine, with 0.5 mg sodium metabisulfite as an antioxidant and 0.2 mg citric acid as a stabilizer. Xylocaine with Epinephrine in multiple dose vials: Each mL also contains 1 mg methylparaben as antiseptic preservative. The pH of these solutions is adjusted to approximately 4.5 (3.3 to 5.5) with sodium hydroxide and/or hydrochloric acid. Filled under nitrogen.
