Dorzolamide Hydrochloride and Timolol Maleate
These highlights do not include all the information needed to use DORZOLAMIDE HYDROCHLORIDE-TIMOLOL MALEATE OPHTHALMIC SOLUTION (preservative-free) safely and effectively. See full prescribing information for DORZOLAMIDE HYDROCHLORIDE-TIMOLOL MALEATE OPHTHALMIC SOLUTION (preservative-free).DORZOLAMIDE HYDROCHLORIDE-TIMOLOL MALEATE ophthalmic solution (preservative-free)Initial U.S. Approval: 1998
4e90c721-21e8-46ea-ab49-19f535bf7202
HUMAN PRESCRIPTION DRUG LABEL
Sep 10, 2025
Akorn
DUNS: 117696873
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Dorzolamide Hydrochloride and Timolol Maleate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
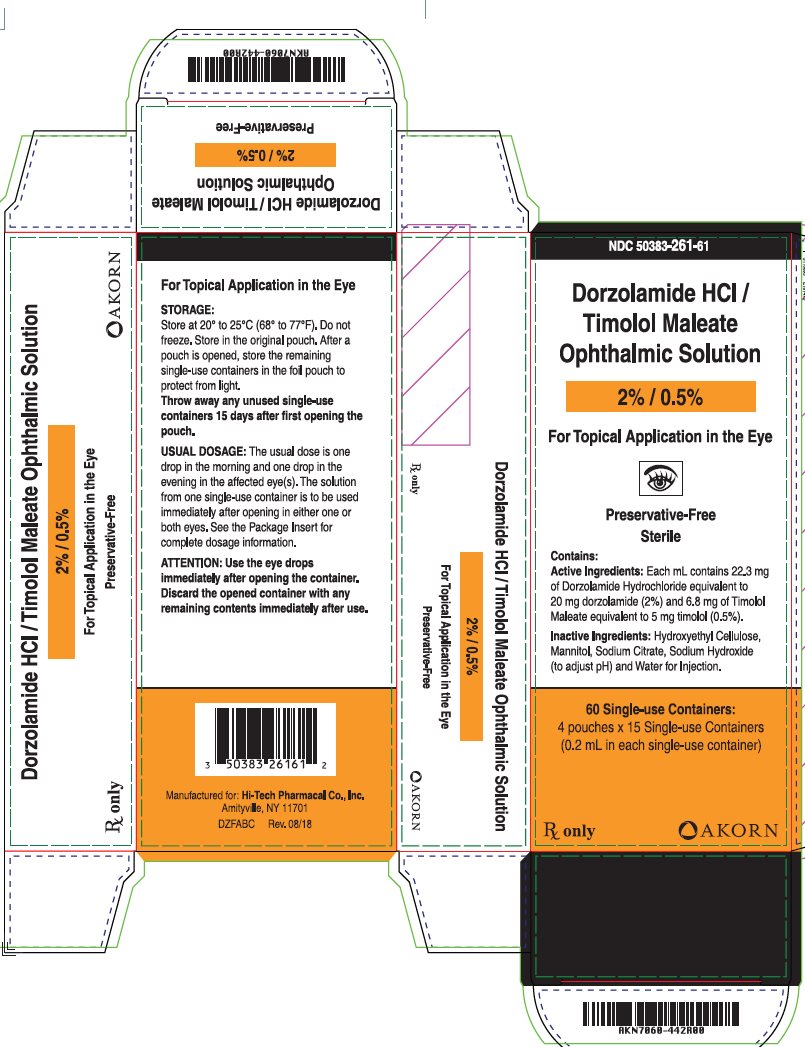
NDC 50383-261-61
Dorzalamide HCl/Timolol Maleate Ophthalmic Solution
2%/5%
For Topical Application in the Eyes
Preservative-Free
Sterile
Contains:
Active Ingredients: Each mL contains 22.3 mg of Dorzolamide Hydrochloride equivalent to 20 mg dorzolamide (2%) and 6.8 mg of Timolol Maleate equivalent to 5 mg timolol (0.5%).
Inactive Ingredients: Hydroxyethyl Cellulose, Mannitol, Sodium Citrate, Sodium Hydroxide (to adjust pH) and Water for injection.
60 Single-use Containers:
4 pouches x 15 Single-use Containers
(0.2 mL in each single-use container)
Rx only
DESCRIPTION SECTION
11 DESCRIPTION
Dorzolamide Hydrochloride-Timolol Maleate Ophthalmic Solution 2%/0.5% (Preservative-Free) is the combination of a topical carbonic anhydrase inhibitor and a topical beta-adrenergic receptor blocking agent.
Dorzolamide hydrochloride is described chemically as: (4S-trans)-4- (ethylamino)-5,6-dihydro-6-methyl-4H-thieno [2,3-b]thiopyran-2-sulfonamide 7,7-dioxide monohydrochloride. Dorzolamide hydrochloride is optically active. The specific rotation is:
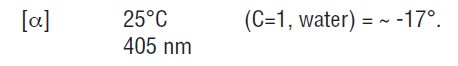
Its empirical formula is C10H16N2O4S3•HCl and its structural formula is:
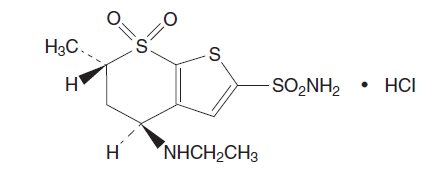
Dorzolamide hydrochloride has a molecular weight of 360.91. It is a white to off-white, crystalline powder, which is soluble in water and slightly soluble in methanol and ethanol.
Timolol maleate is described chemically as: (-)-1-(tert-butylamino)-3- [(4-morpholino-1,2,5-thiadiazol-3-yl)oxy]-2-propanol maleate (1:1) (salt). Timolol maleate possesses an asymmetric carbon atom in its structure and is provided as the levo-isomer. The optical rotation of timolol maleate is:

Its molecular formula is C13H24N4O3S•C4H4O4 and its structural formula is:
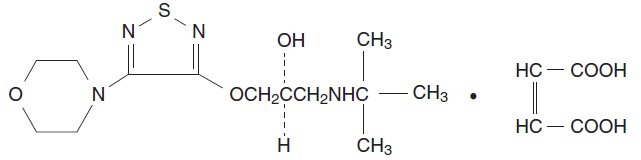
Timolol maleate has a molecular weight of 432.50. It is a white, odorless, crystalline powder which is soluble in water, methanol, and alcohol. Timolol maleate is stable at room temperature.
Dorzolamide Hydrochloride-Timolol Maleate Ophthalmic Solution 2%/0.5% (Preservative-Free) is supplied as a sterile, clear, colorless to nearly colorless, isotonic, buffered, slightly viscous, aqueous solution. The pH of the solution is approximately 5.65, and the osmolarity is 242-323 mOsM. Each mL of Dorzolamide Hydrochloride-Timolol Maleate Ophthalmic Solution 2%/0.5% (Preservative-Free) contains 20 mg dorzolamide (22.26 mg of dorzolamide hydrochloride) and 5 mg timolol (6.83 mg timolol maleate). Inactive ingredients are sodium citrate, hydroxyethyl cellulose, sodium hydroxide, mannitol, and water for injection.
Dorzolamide Hydrochloride-Timolol Maleate Ophthalmic Solution 2%/0.5% (Preservative-Free) does not contain a preservative.
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
See FDA-Approved Patient Labeling (Patient Information).
17.1 Potential for Exacerbation of Asthma and COPD
Dorzolamide hydrochloride-timolol maleate ophthalmic solution (preservative- free) may cause severe worsening of asthma and COPD symptoms including death due to bronchospasm. Patients with bronchial asthma, a history of bronchial asthma, severe chronic obstructive pulmonary disease should be advised not to take this product. [See Contraindications (4.1).]
17.2 Potential of Cardiovascular Effects
Dorzolamide hydrochloride-timolol maleate ophthalmic solution (preservative- free) may cause worsening of cardiac symptoms. Patients with sinus bradycardia, second or third degree atrioventricular block, or cardiac failure should be advised not to take this product. [See Contraindications (4.2).]
17.3 Sulfonamide Reactions
Dorzolamide hydrochloride-timolol maleate ophthalmic solution (preservative- free) contains dorzolamide (which is a sulfonamide) and, although administered topically, is absorbed systemically. Therefore the same types of adverse reactions that are attributable to sulfonamides may occur with topical administration, including severe skin reactions. Patients should be advised that if serious or unusual reactions or signs of hypersensitivity occur, they should discontinue the use of the product and seek their physician’s advice. [See Warnings and Precautions (5.3).]
17.4 Handling the Single-Use Container
Dorzolamide hydrochloride-timolol maleate ophthalmic solution (preservative- free) is a sterile solution that does not contain a preservative. The solution from one individual unit is to be used immediately after opening for administration to one or both eyes. Since sterility cannot be maintained after the individual unit is opened, the remaining contents should be discarded immediately after administration.
17.5 Intercurrent Ocular Conditions
Patients also should be advised that if they have ocular surgery or develop an intercurrent ocular condition (e.g., trauma or infection), they should immediately seek their physician’s advice concerning the continued use of this product.
17.6 Concomitant Topical Ocular Therapy
If more than one topical ophthalmic drug is being used, the drugs should be administered at least five minutes apart.
Manufactured for:Hi-Tech Pharmacal Co., Inc.
Amityville, NY 11701
DZF00N Rev. 08/18
AK60400
SPL UNCLASSIFIED SECTION
Patient Information
Dorzolamide Hydrochloride-Timolol Maleate
(dor-ZOLE-a-mide hye-droe-KLOHR-ide
TIM-uh-lawl MAL-ee-eyt)
Ophthalmic Solution 2%/0.5% (Preservative-Free)
Read this information before you start using dorzolamide hydrochloride-timolol maleate ophthalmic solution (preservative-free) and each time you get a refill. There may be new information. This information does not take the place of talking to your doctor about your medical condition or your treatment.
What is dorzolamide hydrochloride-timolol maleate ophthalmic solution (preservative-free)?
Dorzolamide hydrochloride-timolol maleate ophthalmic solution (preservative- free) is a prescription sterile eye drop solution that contains 2 medicines, dorzolamide hydrochloride (a sulfonamide carbonic anhydrase inhibitor) and timolol maleate (a beta-adrenergic blocker). Dorzolamide hydrochloride-timolol maleate ophthalmic solution (preservative-free) is used to lower the pressure in the eye (intraocular pressure) in people with open-angle glaucoma or ocular hypertension, when their eye pressure is too high and beta-adrenergic blocker medicines alone have not adequately lowered the pressure.
It is not known if dorzolamide hydrochloride-timolol maleate ophthalmic solution (preservative-free) is safe and effective in children under 2 years of age.
Who should not use dorzolamide hydrochloride-timolol maleate ophthalmic solution (preservative-free)?
Do not use dorzolamide hydrochloride-timolol maleate ophthalmic solution (preservative-free) if you:
•
have or have had asthma
•
have or have had severe lung problems (chronic obstructive pulmonary disease)
•
have heart problems, including slow or irregular heartbeat or heart failure
•
are allergic to dorzolamide hydrochloride, timolol maleate, or any of the ingredients in dorzolamide hydrochloride-timolol maleate ophthalmic solution (preservative-free).
Talk to your healthcare provider before taking this medicine if you have any of these conditions.
What should I tell my doctor before using dorzolamide hydrochloride-timolol maleate ophthalmic solution (preservative-free)?
Before you use dorzolamide hydrochloride-timolol maleate ophthalmic solution (preservative-free), tell your doctor if you:
•
have problems with muscle weakness (myasthenia gravis)
•
have diabetes or problems with low blood sugar (hypoglycemia)
•
have thyroid, kidney, or liver problems
•
are planning to have surgery
•
are allergic to sulfa drugs
•
have or have had eye problems, including any surgery on your eye or eyes, or are using any other eye medicines
•
have any other medical problems
•
are pregnant or plan to become pregnant. It is not known if dorzolamide hydrochloride-timolol maleate ophthalmic solution (preservative-free) will harm your unborn baby. If you become pregnant while using dorzolamide hydrochloride-timolol maleate ophthalmic solution (preservative-free) talk to your doctor right away.
•
are breastfeeding or plan to breastfeed. It is not known whether dorzolamide passes into your
breast milk however, timolol has been detected in breast milk. Talk to your doctor about the best
way to feed your baby if you use dorzolamide hydrochloride-timolol maleate ophthalmic solution
(preservative-free).
**Tell your doctor about all the medicines you take,** including prescription and non-prescription medicines,
vitamins, and herbal supplements.
Dorzolamide hydrochloride-timolol maleate ophthalmic solution (preservative-free) and other medicines may affect each other causing side effects. Dorzolamide hydrochloride-timolol maleate ophthalmic solution (preservative-free) may affect the way other medicines work, and other medicines may affect how dorzolamide hydrochloride-timolol maleate ophthalmic solution (preservative-free) works.
Know the medicines you take. Keep a list of them to show your doctor and pharmacist when you get a new medicine.
**How should I use dorzolamide hydrochloride-timolol maleate ophthalmic solution (preservative-free)?**
Read the Instructions for Use at the end of this Patient Information leaflet for additional instructions about the right way to use dorzolamide hydrochloride-timolol maleate ophthalmic solution (preservative-free).
What are the possible side effects of dorzolamide hydrochloride-timolol maleate ophthalmic solution (preservative-free)?
Dorzolamide hydrochloride-timolol maleate ophthalmic solution (preservative- free) may cause serious side effects including:
•
**severe breathing problems.** These breathing problems can happen in people who have asthma, chronic obstructive pulmonary disease, or heart failure and can cause death. Tell your doctor right away if you have breathing problems while taking dorzolamide hydrochloride-timolol maleate ophthalmic solution (preservative-free).
•
**heart failure.** This can happen in people who already have heart failure and in people who have never had heart failure before. Tell your doctor right away if you get any of these symptoms of heart failure while taking dorzolamide hydrochloride-timolol maleate ophthalmic solution (preservative-free):
•
shortness of breath
•
irregular heartbeat (palpitations)
•
swelling of your ankles or feet
•
sudden weight gain
•
**severe allergic reactions.** These allergic reactions can happen the first time you use dorzolamide hydrochloride-timolol maleate ophthalmic solution (preservative-free) or after you have been using dorzolamide hydrochloride-timolol maleate ophthalmic solution (preservative-free) for a while and may cause death.**Stop taking dorzolamide hydrochloride-timolol maleate ophthalmic solution (preservative-free) and call your doctor right away or get emergency help if you get any of these symptoms of an allergic reaction**
•
swelling of your face, lips, mouth, or tongue
•
trouble breathing
•
wheezing
•
severe itching
•
skin rash, redness, or swelling
•
dizziness or fainting
•
fast heartbeat or pounding in your chest (tachycardia)
•
sweating
•
**worsening muscle weakness.** Dorzolamide hydrochloride-timolol maleate ophthalmic solution (preservative-free) can cause muscle weakness to get worse in people who already have problems with muscle weakness (myasthenia gravis).
•
**kidney problems.** Your doctor may do tests to check your kidney function while you use dorzolamide hydrochloride-timolol maleate ophthalmic solution (preservative-free).
•
swelling of your eye (cornea)
The most common side effects of dorzolamide hydrochloride-timolol maleate ophthalmic solution (preservative-free) include:
•
a bitter, sour, or unusual taste in your mouth after using dorzolamide hydrochloride-timolol maleate ophthalmic solution (preservative-free)
•
burning, stinging, redness, or itching of the eye
•
blurred vision
•
painful, red, watery eyes with increased sensitivity (superficial punctate keratitis)
Tell your doctor if you have any new eye problems while using dorzolamide hydrochloride-timolol maleate ophthalmic solution (preservative-free) including:
•
an eye injury
•
an eye infection
•
a sudden loss of vision
•
eye surgery
•
swelling and redness of and around your eye (conjunctivitis)
•
problems with your eyelids
Tell your doctor if you have any other side effects that bother you.
These are not all the possible side effects of dorzolamide hydrochloride- timolol maleate ophthalmic solution (preservative-free). For more information, ask your doctor or pharmacist.
Call your doctor about medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
What should I do in case of an overdose?
If you swallow the contents of the container, contact your doctor immediately. Among other effects, you may feel light-headed, have difficulty breathing, or feel your heart rate has slowed.
How should I store dorzolamide hydrochloride-timolol maleate ophthalmic solution (preservative-free)?
•
Store dorzolamide hydrochloride-timolol maleate ophthalmic solution (preservative-free) at room temperature between 68°F to 77°F (20°C to 25°C). Do not freeze.
•
Keep the dorzolamide hydrochloride-timolol maleate ophthalmic solution (preservative-free) single-use containers in their original foil pouch to protect from light.
•
Write down the date you open the foil pouch in the space provided on the pouch.
•
Throw away all unused dorzolamide hydrochloride-timolol maleate ophthalmic solution (preservative-free) single-use containers 15 days after first opening the pouch.
Keep dorzolamide hydrochloride-timolol maleate ophthalmic solution (preservative-free) and all medicines out of the reach of children.
General information about the safe and effective use of dorzolamide hydrochloride-timolol maleate ophthalmic solution (preservative-free).
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use dorzolamide hydrochloride-timolol maleate ophthalmic solution (preservative-free) for a condition for which it was not prescribed. Do not give dorzolamide hydrochloride-timolol maleate ophthalmic solution (preservative-free) to other people, even if they have the same symptoms you have. It may harm them. This Patient Information leaflet summarizes the most important information about dorzolamide hydrochloridetimolol maleate ophthalmic solution (preservative-free). If you would like more information, talk with your doctor. You can ask your pharmacist or doctor for information about dorzolamide hydrochloride-timolol maleate ophthalmic solution (preservative-free) that is written for health professionals.
What are the ingredients in dorzolamide hydrochloride-timolol maleate ophthalmic solution (preservative-free)?
Active ingredients: dorzolamide hydrochloride and timolol maleate
Inactive ingredients: sodium citrate, hydroxyethyl cellulose, sodium hydroxide, mannitol, and water for injection.
Instructions for Use
Read these instructions before using your dorzolamide hydrochloride-timolol maleate ophthalmic solution (preservative-free) and each time you get a refill. There may be new information. This leaflet does not take the place of talking with your doctor about your medical condition or your treatment.
Important:
•
Dorzolamide hydrochloride-timolol maleate ophthalmic solution (preservative-free) is for the eye only. Do not swallow dorzolamide hydrochloride-timolol maleate ophthalmic solution (preservative-free).
•
Dorzolamide hydrochloride-timolol maleate ophthalmic solution (preservative-free) single-use containers are packaged in a foil pouch.
•
Write down the date you open the foil pouch in the space provided on the pouch.
Every time you use dorzolamide hydrochloride-timolol maleate ophthalmic solution (preservative-free):
|
Step 1. Wash your hands. | |
|
Step 2. Take the strip of single-use containers from the pouch. | |
|
Step 3. Pull off 1 single-use container from the strip. | |
|
Step 4. Put the remaining strip of single-use containers back in the pouch and fold the edge to close the pouch. | |
|
Step 5. Hold the single-use container upright. Make sure that the solution is in the bottom part of the single- use container**(See Figure A).** |
|
|
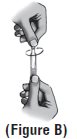
Step 6. Open the single-use container by twisting off the tab**(See Figure B).** |
|
|
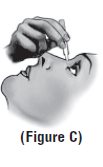
Step 7. Tilt your head backwards. If you are unable to tilt your head, lie down. Step 8. Place the tip of the single-use container close to your eye. Be careful not to touch your eye with the tip of the single-use container**(See Figure C).** |
|
|
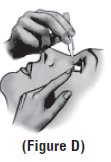
Step 9. Pull the lower eyelid downwards and look up. Step 10. Gently squeeze the container and let 1 drop of Dorzolamide hydrochloride-timolol maleate ophthalmic solution (preservative-free) fall into the space between your lower eyelid and your eye. If a drop misses your eye, try again**(See** Figure D). |
|
|
Step 11. Blot any excess solution from the skin around the eye with a tissue. |
•
If your doctor has told you to use drops in both eyes, repeat steps 7 to 11 for your other eye.
•
There is enough dorzolamide hydrochloride-timolol maleate ophthalmic solution (preservative-free) in 1 single-use container for 1 or both of your eyes.
•
**Throw away the opened single-use container with any remaining dorzolamide hydrochloride-timolol maleate ophthalmic solution (preservative-free) right away.**
This Patient Information and Instructions for Use have been approved by the U.S. Food and Drug Administration.
Manufactured for:Hi-Tech Pharmacal Co., Inc.
Amityville, NY 11701
DZFA0N Rev. 08/18
AK60400