Phenohytro
PHENOHYTRO ELIXIR
a5aaf1db-1938-4073-9da4-c47f959d013e
HUMAN PRESCRIPTION DRUG LABEL
Sep 22, 2025
ATLANTIC BIOLOGICALS CORP.
DUNS: 047437707
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Phenobarbital, Hyoscyamine Sulfate, Atropine sulfate, and Scopolamine Hydrobromide
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (23)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL -
NDC 17856-0125-01
Phenohytro ®
Elixir 10mL CUP
Grape Flavored
Each 5 mL (1 teaspoonful) contains:
Phenobarbital, USP
16.2 mg
Hyoscyamine Sulfate, USP
0.1037 mg
Atropine Sulfate, USP
0.0194 mg
Scopolamine Hydrobromide, USP
0.0065 mg
Alcohol not more than 23.8%
DO NOT USE IF TAMPER-EVIDENT SEAL
UNDER CAP IS BROKEN OR MISSING
DEA EXEMPTRx Only

CONTRAINDICATIONS SECTION
CONTRAINDICATIONS
PHENOHYTRO ®ELIXIR is contraindicated in patients with known hypersensitivity to any of the ingredients. Phenobarbital is contraindicated in patients with acute intermittent porphyria and in those patients in which phenobarbital produces restlessness and/or excitement.
PHENOHYTRO ®ELIXIR is also contraindicated in patients with glaucoma, obstructive uropathy; paralytic ileus; myasthenia gravis; intestinal atony; unstable cardiovascular status in acute hemorrhage; severe ulcerative colitis especially if complicated by toxic megacolon; hiatal hernia associated with reflux esophagitis; obstructive disease of the gastrointestinal tract; or severe ulcerative colitis.
ADVERSE REACTIONS SECTION
ADVERSE REACTIONS
Call your doctor for medical advice about side effects.
Adverse reactions associated with anticholinergics and/or anticonvulsants are: dry mouth; tachycardia; urinary hesitancy and retention; palpitation; blurred vision; prolonged pupil dilation; cycloplegia; increased ocular tension; loss of taste sense; headache; nervousness; drowsiness; weakness; dizziness; insomnia; nausea; vomiting; severe allergic reaction or drug idiosyncrasies, including anaphylaxis, hives and/or other dermal manifestations; decreased sweating; impotence; suppression of lactation; constipation; bloated feeling and musculoskeletal pain. Elderly patients may react with symptoms of excitement, agitation and drowsiness to even small doses of the drug. Phenobarbital may produce excitement in some patients, rather than a sedative effect. In patients habituated to barbiturates, abrupt withdrawal may produce delirium or convulsions.
To report SUSPECTED ADVERSE REACTIONS, contact FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DESCRIPTION SECTION
DESCRIPTION
PHENOHYTRO® ELIXIR Mint Flavored
each 5 mL (teaspoonful) oral-administered dose of elixir contains:
|
Phenobarbital, USP (**WARNING:**may be habit forming) |
16.2 mg |
|
Hyoscyamine Sulfate, USP |
0.1037 mg |
|
Atropine Sulfate, USP |
0.0194 mg |
|
Scopolamine Hydrobromide, USP |
0.0065 mg |
|
Alcohol not more than 23.8% |
INACTIVE INGREDIENTS
Purified water, glycerin, sorbitol, ethyl alcohol, sucrose, sodium saccharin, artificial mint flavor, FD&C Yellow No. 5, FD&C Blue No. 1.
Phenobarbital is a barbiturate with the chemical name 2,4,6(1H,3H,5H) -Pyrimidinetrione, 5-ethyl-5-phenyl-. It has the following structural formula:
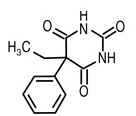
C12H12N2O3 Chemical Structure M.W. 232.2
Hyoscyamine sulfate is a belladonna alkaloid with the chemical name Benzeneacetic acid, α-(hydroxmethyl)-, 8-methyl-8-azabicyclo[3.2.1]oct-3-yl ester, [3(S)-endo]-, sulfate (2:1), dihydrate. It has the following structural formula:
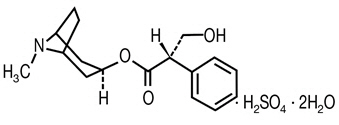
(C17H23NO3)2 ∙ H2SO4 ∙ 2H2O Chemical Structure M.W. 712.85
Atropine sulfate is belladonna alkaloid with the chemical name: Benzeneacetic acid, α-(Hydroxymethyl)benzeneacetic acid 8-methyl-8-azabicyclo[3.2.1]oct-3-yl ester. It has the following structural formula:
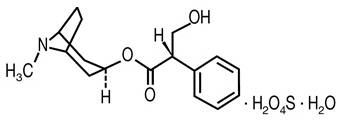
(C34H46N2O6 ∙ H2O4S ∙ H2O Chemical Structure M.W. 694.83
Scopolamine hydrobromide is a belladonna alkaloid with the chemical name Benezeneacetic acid, α-(hydroxymethyl)-, 9-methyl-3-oxa-9-azatricyclo[3.31.0.2,4]non-[7-yl ester, hydrobromide, trihydrate, [7(S)-(1α,2β,4β,5α,7β)]-. It has the following structural formula:
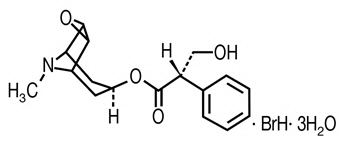
C17H21NO4 ∙ BrH ∙ 3H2O Chemical Structure M.W. 438.31
WARNINGS SECTION
WARNINGS
Heat prostration can occur with belladonna alkaloids in high temperatures.
Diarrhea may be an early symptom of incomplete intestinal obstruction, particularly in patients with ileostomy or colostomy. In this instance, treatment with this drug could be harmful.
PHENOHYTRO ®ELIXIR may produce drowsiness and blurred vision. The patient should be warned about engaging in hazardous work or activities requiring mental alertness, such as operating a motor vehicle or other machinery.
Phenobarbital may decrease the effect of anticoagulants, and larger doses of the anticoagulant may be necessary for optimal effect. When phenobarbital is discontinued, the dose of the anticoagulant may have to be decreased.
Phenobarbital may be habit formingand should not be administered to patients who are susceptible to addiction or to those with a history of physical and/or psychological drug dependence.
Barbiturates should be used with caution in patients with hepatic dysfunction.
PRECAUTIONS SECTION
PRECAUTIONS
GENERAL
Use with caution in patients with: autonomic neuropathy, hepatic or renal disease, hyperthyroidism, coronary heart disease, congestive heart failure, cardiac arrhythmias, tachycardia, and hypertension.
Belladonna alkaloids may produce a delay in gastric emptying (antral stasis), which would complicate the management of gastric ulcer.
Do not rely on the use of the drug in the presence of complication of biliary tract disease. Theoretically, a curare-like action may occur with overdosage.
CARCINOGENESIS, MUTAGENESIS, IMPAIRMENT OF FERTILITY
Long-term studies in animals have not been performed to evaluate carcinogenic potential.
PREGNANCY
PREGNANCY CATEGORY C
Animal reproduction studies have not been conducted with PHENOHYTRO ®ELIXIR. It is not known whether PHENOHYTRO ®ELIXIR can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. PHENOHYTRO ®ELIXIR should be given to a pregnant woman only if clearly needed.
NURSING MOTHERS
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, exercise caution when administering PHENOHYTRO ®ELIXIR to a nursing woman.
INFORMATION FOR PATIENTS
Practitioners should give the following information and instructions to patients:
- Do not increase the dose of this drug without consulting a physician.
- Do not share this medication with others.
- The use of this product carries with it an associated risk of psychological and/or physical dependence.
- The use of this product may impair mental and/or physical abilities required for the performance of potentially hazardous tasks such as driving or operating machinery.
- Use of this product with alcohol may result in additional central nervous system depressant effects.
- Tell your doctor or pharmacist if you also take antihistamines, anti-seizure drugs, medicine for sleep or anxiety, muscle relaxants, narcotic pain relievers, or psychiatric medicines.
- This drug may increase the risk for heatstroke because it decreases sweating. Avoid becoming overheated in hot weather, saunas, and during exercise or other strenuous activity.
OVERDOSAGE SECTION
OVERDOSAGE
The signs and symptoms of overdose are headache, nausea, vomiting, blurred vision, dilated pupils, hot and dry skin, dizziness, dryness of the mouth, difficulty in swallowing, and CNS stimulation. Call your doctor or local Poison Control Center if overdosage is suspected.
The dosage of PHENOHYTRO ®ELIXIR should be adjusted to the needs of the individual patient to assure symptomatic control with a minimum of adverse effects.
DOSAGE & ADMINISTRATION SECTION
DOSAGE AND ADMINISTRATION
Adults
One or two teaspoonfuls of PHENOHYTRO ®ELIXIR three or four times a day according to conditions and severity of symptoms.
Pediatric patients
may be dosed every 4 to 6 hours.
Starting Dosage|
Body Weight |
q4h |
q6h |
|---|---|---|
|
10 lb. (4.5 kg) |
0.5 mL |
0.75 mL |
|
20 lb. (9.1 kg) |
1.0 mL |
1.5 mL |
|
30 lb. (13.6 kg) |
1.5 mL |
2.0 mL |
|
50 lb. (22.7 kg) |
1/2 tsp |
3/4 tsp |
|
75 lb. (34 kg) |
3/4 tsp |
1 tsp |
|
100 lb. (45.4kg) |
1 tsp |
1 1/2 tsp |
HOW SUPPLIED SECTION
HOW SUPPLIED
PHENOHYTRO**®****ELIXIR Grape Flavored**is a purple colored, grape flavored liquid.
NDC 17856-0125-01 Grape Flavored in 10mL 72 CUP
NDC 17856-0125-02 Grape Flavored in 5mL 72 CUP
STORAGE CONDITIONS
AVOID FREEZING
Store PHENOHYTRO ®ELIXIR at 20° - 25°C (68° - 77°F) [see USP Controlled Room Temperature].
Protect from light and moisture.
Dispense in a tight, light-resistant container as defined in the USP using a child-resistant closure. Use safety closures when dispensing this product unless otherwise directed by a physician or requested by purchaser.
WARNINGS: KEEP THIS AND ALL DRUGS OUT OF THE REACH OF CHILDREN.
IN THE CASE OF OVERDOSE, SEEK PROFESSIONAL ASSISTANCE OR CONTACT A POISON CONTROL CENTER IMMEDIATELY.
Contains color additives, including FD&C Yellow No. 5 (tartrazine).
